Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 5 - BINDING
E: MODERN METHODS IN DRUG DEVELOPMENT
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Updated: 03/31/16
|
Learning Goals/Objectives for Chapter 5E: After class and this reading, students will be able to
|
E8. Multivalent Inhibitors
In the previous study guide on binding and transcription, we discussed how a protein might bind DNA cooperatively if it interacts with a pre-bound protein adjacent to its normal binding site as well as its own binding site. The protein-protein interaction effectively reduces the apparent koff of the protein for its DNA binding site, which decreases the apparent Kd. Imagine a variation on this. Consider what might happen if a ligand for a macromolecule receptor found on the cell membrane was present in multiple copies. That is, what if the ligand was multivalent? What if the receptor was also multivalent? Both these cases are often found when complex ligands (bearing complex carbohydrates, for example) are present on the presenting ligand (a virus or bacteria, for example) and the macromolecule receptor is a cell surface protein. An interaction between the multivalent ligand and the receptor is necessary for the initial biological interaction between them.
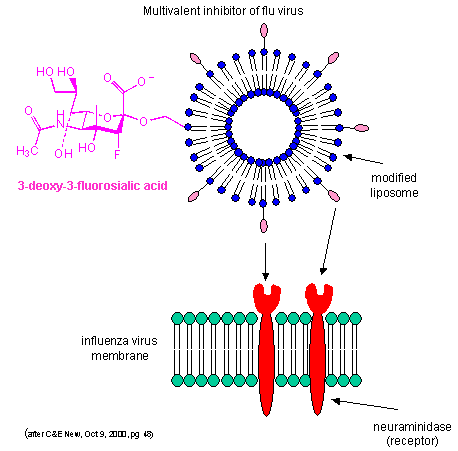
By decreasing the apparent koff as described above, multiple weak binding interactions can be effectively turned into strong interactions. Creating multivalent drug inhibitors of viral or bacterial interactions with cell membrane receptors would increase their efficacy. For example, consider the binding of the influenza virus to its receptor on a cell. Two viral proteins, hemagglutinin, as well as neuraminidase, binds to sialic acid residues on the glycoproteins receptors of the cells. A single, monovalent sialic acid derivative (acting as a drug) would be a low affinity inhibitor of the viral-cell interaction. A multivalent inhibitor, in which multiple sialic acid derivatives were covalently attached to a liposome proved to be a much more effective inhibitor since it could bind to multiple hemagglutinin and neuramindase molecules on the viral surface with an apparent lower Kd. This inhibitor is 1000X as potent as the monovalent inhibitor. This technique can be used not only to inhibit, but also activate biological reactions.
Figure: Multivalent inhibitor of flu virus
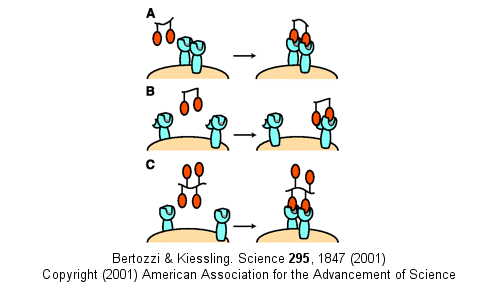
Other mechanism can also account for the amplified effects of multivalent ligands in addition to the effects on koff (which is related to the effective local concentration of the ligand at the receptor site - an effect also called the chelate effect). These include ligand-induced clustering of receptors on the cell surface and binding of multivalent ligands to more than one site or subsite on the receptor. These are illustrated below.
Figure: Multivalent Ligand Binding Models
Navigation
Return to 5E. Modern Methods in Drug Development Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 5E: Modern Methods in Drug Development

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.