Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 7 - CATALYSIS
A: METHODS OF CATALYSIS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 04/11/16
|
Learning Goals/Objectives for Chapter 7A: After class and this reading, students will be able to
|
Reactions in solution that are not catalyzed are slow since charge development and separation occurs in the transition state. When bonds are made or broken, charged intermediates are often formed which are higher in energy than the reactants. Since the intermediate is higher in energy than the reactants, the transition state would be even higher in energy, and hence more closely resemble the charged intermediate. Anything that can stabilize the charges on the intermediate and hence the developing charges in the transition states will lower the energy of the transition state and catalyze the reaction. In this section will will investigate the mechanism underlying the catalysis by small molecules of chemical reactions. Presumably, biological macromolecular catalyst (like protein enzymes) will use similar mechanisms in their catalytic effects (which will be discussed in the next section).
Catalysts, including enzymes, can employ at least 5 different ways to stabilize transition states.
A1. General Acid and Base Catalysis
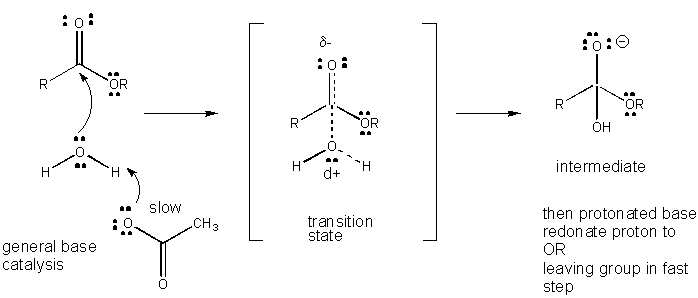
Charge development in the TS can be decreased by either donation of a proton from general acids (like acetic acid or a protonated indole ring) to an atom such as a carbonyl O which develops a partial negative charge in the TS when it is attached by a nucleophile. Proton donation decreases the developing negative in the TS. Alternatively, a nucleophile such as water which develops a partial positive charge in the TS as it begins to form a bond to an electrophilic C in a carbonyl can be stabilized by the presence of a general base (such as acetate or the deprotonated indole ring). Proton abstraction decreases the developing positive charge
Figure: CHARGE DEVELOPMENT IN THE TRANSITION STATE FOR ESTER HYDROLYSIS
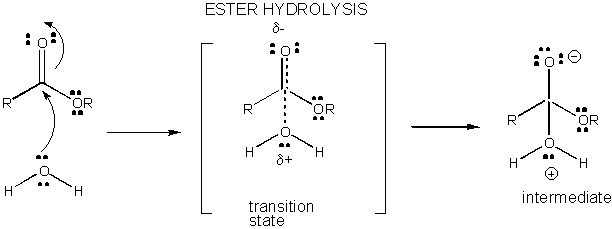
Figure: MECHANISM OF GENERAL ACID CATALYSIS
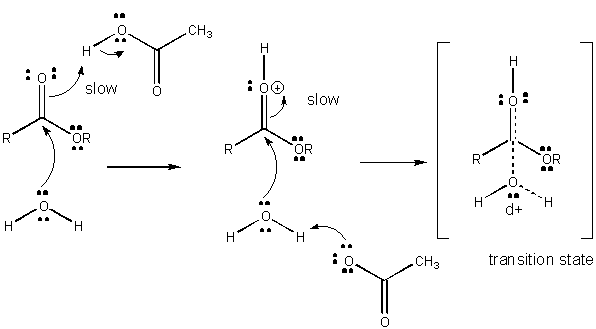
Figure: MECHANISM OF GENERAL BASE CATALYSIS
Navigation
Return to 7A: Methods of Catalysis Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 7A: Methods of Catalysis

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.