Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 7 - CATALYSIS
A: METHODS OF CATALYSIS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 04/11/16
|
Learning Goals/Objectives for Chapter 7A: After class and this reading, students will be able to
|
A2. Metal Ion or Electrostatic Catalysis
A metal such as Cu2+ or Zn2+ can also stabilize the TS. The metal must be able to be bound to the charged intermediate and hence the TS. The tetrahedral oxyanion intermediate of the reaction of an electrophilic carbonyl C can interact with a metal if there is an O on an adjacent atom which can help coordinate the metal ion. This charge stabilization of the developing negative in the TS and the full negative in the intermediate is often called electrostatic catalysis. This method is likely to be found in many enzymes since nearly 1/3 of all enzymes require metal ions. A classic example of an enzyme using metal ion catalysis is carboxypeptidase A.
Figure:
METAL ION OR ELECTROSTATIC CATALYSIS - STABILIZATION OF TS CHARGE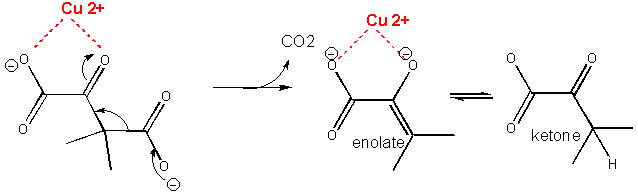
Metals can also act in a different way. They may coordinate a water and by further polarizing the H-O bond increase the acidity of the bound water. For instance, the water molecule in the pentammineaquacobalt(III) ion has a pKa of 6.6, compared to pure water, with a pKa of 15.7. (To calculate the latter, write the equilibrium expression for water: H2O + H2O <==> H3O+ + OH- . Then write the Ka expression, as for a generic acid, which is [H3O+][ OH-]/ [H2O] = 10-14/55.5. The pKa is 15.7). The complexed hydroxide is a better nucleophile than bulk water. An example of an enzyme whose bound metal increases the nucleophilicity of water is carbonic anhydrase.
Figure: METAL ION CATALYSIS - DECREASING pKa OF WATER
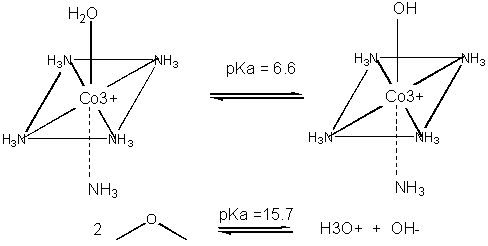
Navigation
Return to 7A: Methods of Catalysis Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 7A: Methods of Catalysis

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.