Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 7 - CATALYSIS
A: METHODS OF CATALYSIS
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 04/11/16
|
Learning Goals/Objectives for Chapter 7A: After class and this reading, students will be able to
|
A3. Covalent or Nucleophilic Catalysis
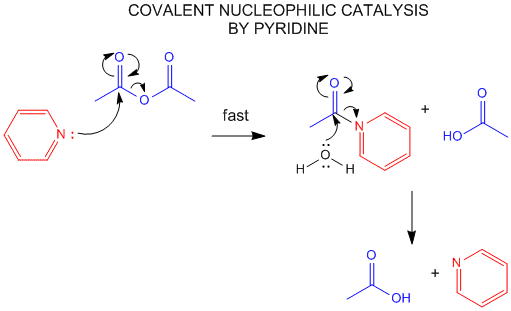
One way to change the activation energy of the reaction is to change the reaction mechanism in ways which introduces new steps with lower activation energy. A typical way is to add a nucleophilic catalyst which forms a covalent intermediate with the reactant. The original nucleophile can then interact with the intermediate in a nucleophilic substitution reaction. If the nucleophilic catalyst is a better nucleophile than the original nucleophile (usually water) then the reaction is catalyzed. The nucleophilic catalyst and the original nucleophile usually interact with a carbonyl C in a substitution reaction, initially forming the tetrahedral oxyanion intermediate.
Figure: NUCLEOPHILIC COVALENT CATALYSIS BY PYRIDINE.
If an amine is used as the nucleophilic catalyst, then the initial addition product (a carbinolamine) can become dehydrated, since the free pair of electrons on the N are more likely to be shared with the carbon to form a double bond than electrons from the original carbonyl O, which is more electronegative than the N). An imine or Schiff Base forms, with a pKa of about 7.
Figure: MECHANISM OF SCHIFF BASE FORMATION
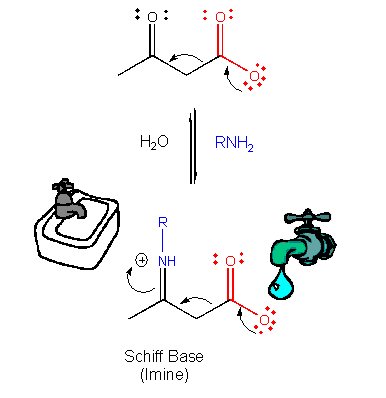
This is easily protonated to form a positively charged N at the former carbonyl O center. This serves as an excellent electron sink for decarboxylation reactions of beta-keto acids and illustrates an important point. Electrons in chemical reactions can be viewed as flowing from a source (such as a carboxyl group) to a sink (such as an nucleophilic carbonyl O or a positively charged N in a Schiff base).
Figure: MECHANISM OF NUCLEOPHILIC CATALYSIS BY AMINES - SCHIFF BASE FORMATION.
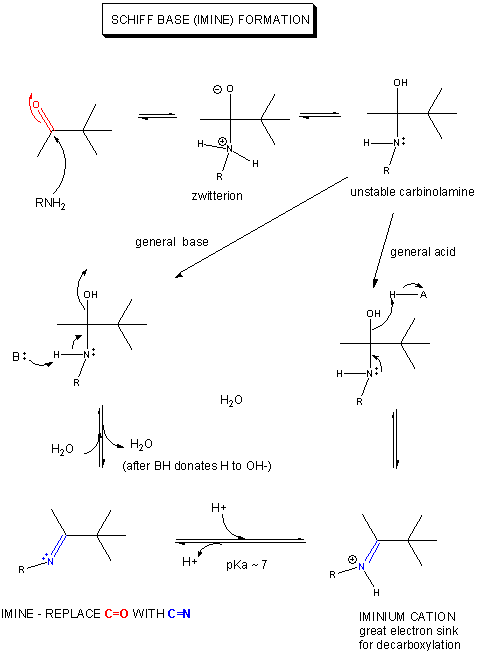
Figure: ELECTRON FLOW: SOURCE TO SINK
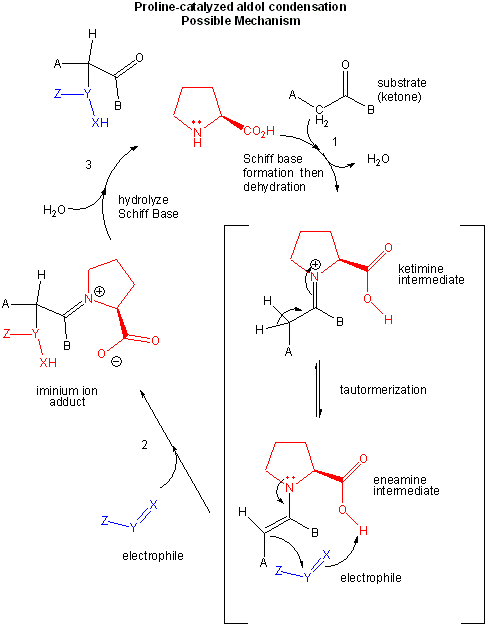
In a subsequent section, we will discuss how protein enzymes use these same catalytic strategies. An intriguing question arises: how much of the structure of a large protein is really needed for catalysis? Much work has been directed to the development of small molecule catalysis mimetics of large protein enzymes. Just how small can you go in reducing the size of a protein and still get catalysis. One important feature of enzyme catalysis is that they catalyze reactions in which only one enantiomer is produced. That is, the synthesis is assymertric. This is typically a consequence of the asymmetric enzyme (itself chiral) binding only one enantiomer as a reactant and/or the imposition of steric restrictions on the possible reactions of the bound substrate. Recently, it has been show that L-Pro alone can act as such an assymetric catalyst in an aldol condensation reaction.
Figure: L-PRO CATALYSIS OF AN ALDOL CONDENSATION: POSSIBLE MECHANISM
Navigation
Return to 7A: Methods of Catalysis Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 7A: Methods of Catalysis

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.