Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
A: Mono- and Disaccharides
03/14/16
|
Learning Goals/Objectives for Chapter 3A: After class and this reading, students will be able to
|
The link below is an extraordinary and free resource on glycobiology. It defines the word "glycan" as a "generic term for any sugar or assembly of sugars, in free form or attached to another molecule" and "is used interchangeably ... with saccharide or carbohydrate."
-
 Essentials
of Glycobiology, 2nd edition, is available online.
Essentials
of Glycobiology, 2nd edition, is available online.
A2. Isomers
Sugars can exists as either configurational isomers (interconverted only by breaking covalent bonds) and conformational isomers. The figure below reviews different types of isomers.
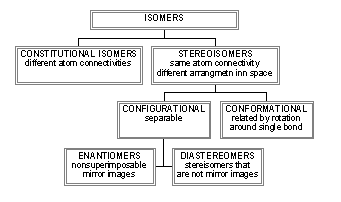
The configurational isomers include enantiomers (stereoisomers that are mirror images of each other), diastereomers (stereoisomers that are not mirror images), epimers (diastereomers that differ at one stereocenter), and anomers (a special form of stereoisomer, diastereomer, and epimer that differ only in the configuration around the carbon which was attacked in the intramolecular nucleophilic attack to produces the a and b isomers).
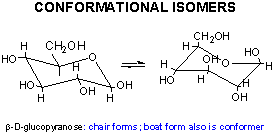
Sugars can also exist as conformational isomers, which interchange without breaking covalent bonds. These include chair and boat conformations of the cyclic sugars.
Navigation
Return to Chapter 3A: Monosaccharide Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3A: Mono- and Disaccharides

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.