Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
A: Mono- and Disaccharides
03/14/16
|
Learning Goals/Objectives for Chapter 3A: After class and this reading, students will be able to
|
The link below is an extraordinary and free resource on glycobiology. It defines the word "glycan" as a "generic term for any sugar or assembly of sugars, in free form or attached to another molecule" and "is used interchangeably ... with saccharide or carbohydrate."
-
 Essentials
of Glycobiology, 2nd edition, is available online.
Essentials
of Glycobiology, 2nd edition, is available online.
A3. Monosaccharide Derivatives
Many derivatives of monosaccharides are found in nature. These include
- oxidized forms in which the aldehyde and/or alcohol functional groups are oxidized to carboxylic acids
- phosporylated forms in which phosphate is added by ATP to form phosphoester derivatives
- amine derivatives such as glucosamine or galactosamine
- acetylated amine derivatives such as N-Acetyl-GlcNAc (GlcNAc) or GalNAc
- lactone forms (intramolecular esters) in which an OH group attacks a carbony C that was previously oxidized to a carboxylic acid
- condensation products of sugar derivatives with lactate (CH3CHOHCO2-) and pyruvate, (CH3COCO2- ), both from the glycolytic pathway, to form muramic acid and neuraminic acids, (also called sialic acids), respectively.
Figure: Sugar Derivatives
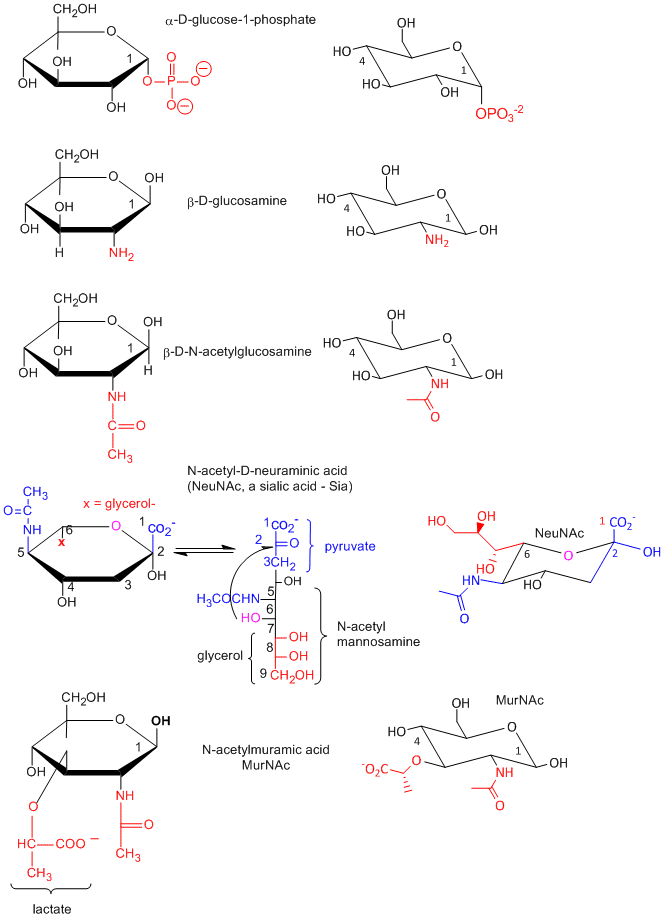
In the above figure, N-acetylmuramic acid, found in bacterial cell walls, consists of GlcNAc in ether link at C3 with lactate, while N-acetylneuraminic acid results from an intramolecular cyclization of a condensation product of ManNAc and pyruvate.
Figure: Is sialic acid the big difference between humans and chimps?
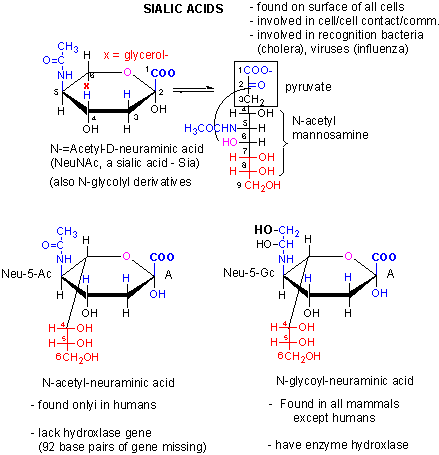
What happens when non-vegan humans eat animal products (meat, milk) with N-glycoyl neuraminic acids (Neu5Gc)? Varki et al found that some gets incorporated into human membrane glycans (see next Chapter). Sialic acids on surface proteins can serve as "receptors" that allowing binding of self-cells as well as foreign cells or proteins that have evolved to bind them. Byres et al discovered that a toxin, SubAB, secreted by E. Coli 0157, can bind Neu5Gc. Hence eating meat products can make us more susceptible to bacteria that recognize Neu5Gc.
Navigation
Return to Chapter 3A: Monosaccharide Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3A: Mono- and Disaccharides

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.