Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
A: Mono- and Disaccharides
03/14/16
|
Learning Goals/Objectives for Chapter 3A: After class and this reading, students will be able to
|
The link below is an extraordinary and free resource on glycobiology. It defines the word "glycan" as a "generic term for any sugar or assembly of sugars, in free form or attached to another molecule" and "is used interchangeably ... with saccharide or carbohydrate."
-
 Essentials
of Glycobiology, 2nd edition, is available online.
Essentials
of Glycobiology, 2nd edition, is available online.
A4. Formation of Hemiacetals, Acetals, and Disaccharides
Monosaccharides that contain aldehydes can cyclize through intramolecular nucleophilic attack of an OH at the carbonyl carbon in an addition reaction to form a hemiacetal (hemiketal if attack on a ketone). On the addition of acid (which protonates the anomeric OH, forming water as a potential leaving group), another alcohol can add forming an acetal (or ketal from a ketone) with water leaving.
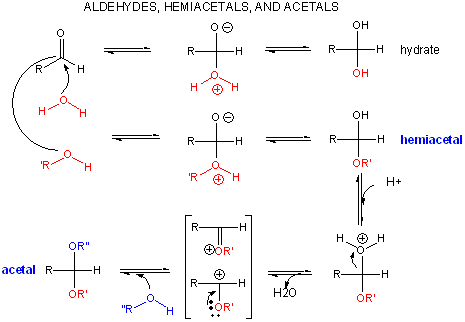
If the other alcohol is a second monosaccharide, a dissacharide results. The acetal (or ketal) link bonding to the two monosaccharides is called a glycosidic link. Links between the two sugars can be either a (if the OH on C1 involved in the glycosidic link is pointing down) or b (if the O on C1 involved in the glycosidic link is pointing up). Since sugars contain so many OH groups which can act as the "second" alcohol in acetal (or ketal) formation, links between sugars can be quite diverse. These include a and b forms of 1-2, 1-3, 1-4, 1-5, 1-6, 2-2, etc. links. For example:
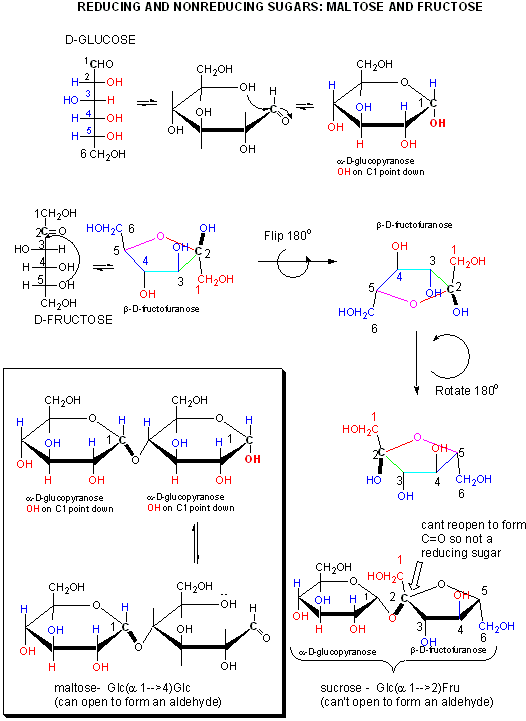
- lactose: Gal(b 1->4)Glc Since Glc is attached to Gal through the OH on C4, its anomeric carbon, C1, could revert to the noncyclic aldehyde form. This aldehyde is susceptible to oxidation by reagents (Benedicts Solution - with citrate, Fehlings reagent - with tartrate) which are subsequently reduced. In both reagents, reducing sugars reduce a basic blue solution of CuSO4 (Cu2+) to form a brick red precipate of Cu2O (Cu+). Sugars (monosaccharides, dissacharides and polysaccharides which can form an aldehyde at C1 or have an a-hydroxymethyl ketone group which can isomerize to an aldehyde under basic conditions (such as fructose) are called reducing sugars. These oxidizing agent are mild and react with aldehydes and not ketones.
- sucrose: Glc(a 1->2)Fru. Since Fru is attached through the anomeric OH of this ketose, the Fru is not in equilibrium with its straight chain keto form, and hence sucrose is a nonreducing sugar.
The glycosidic (acetal or ketal) link can be cleaved by hydrolysis, just as the peptide bond in proteins.
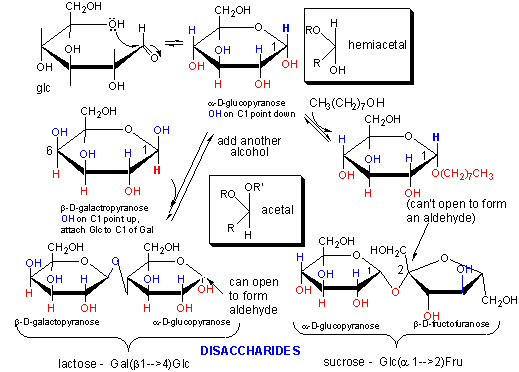
Figure: A closer look at reducing and nonreducing sugars: lactose and maltose
![]()
![]() Jmol:
D
Glucose
Jmol:
D
Glucose
![]()
![]() Jmol:
Acetal Formation
Jmol:
Acetal Formation
Navigation
Return to Chapter 3A: Monosaccharide Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3A: Mono- and Disaccharides

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.