Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 3 - CARBOHYDRATES/GLYCANS
D: Glycoprotein Biosynthesis and Function
01/27/14
|
Learning Goals/Objectives for Chapter 3B: After class and this reading, students will be able to
|
This guide on carbohydrates will review those features that are deemed especially important for a one semester Biochemistry course dealing with structure/function relationships of biological molecules.
D2. Glycoprotein Function
The role of CHO in glycoprotein structure/function is slowly being determined. The most important seems to involve their role in directing proper folding of proteins in the ER which accounts for the observations that glycan addition to proteins in the ER is a cotranslational event. When inhibitors of ER glycosylation are added to cells, protein misfolding and aggregation are observed. The extent of misfolding depends on the particular protein and particular glycosylation sites with the protein. The polar CHO residues help promote solubility of folding intermediates, similar to the effects of many chaperone proteins.
The glycan moieties of the folding glycoprotein also lead to binding of the protein to lectins in the ER which serve as molecular chaperones. The most studied of these chaperones are involved in the calnexin-calreticulin cycle, and facilitate correct disulfide bond formation in the protein. After two glucose residues are removed by glucosidase I and II, the monoglucosylated protein binds to calnexin (CNX) and/or calreticulin (CRT), two homologous ER lectins specific for monoglucosylated proteins. Once bound, another protein, ERp57, a molecular chaperone with a disulfide bond (shown in diagram) interacts with the protein. This protein has protein disulfide isomerase activity.
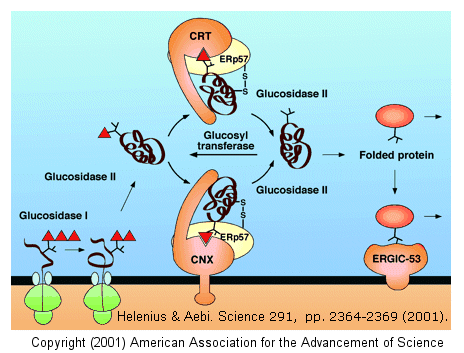
If a glycoprotein has not folded completely, it is recognized by a glycoprotein glucosyltransferase, which adds a glucose to it. This then promotes reentry into the calnexin/calreticulin cycle.
Ideally, unfolded or misfolded proteins would be targeted from degradation and elimination from cells. The ER has evolved a system to accomplish this. Since folding occurs in the ER, to prevent misfolding and aggregation, the ER also contains chaperones and folding catalysts. Stress (such as through heat shock) stimulates ER chaperone activity. As a final defense mechanism, unfolded or aberrantly-folded proteins are degraded by the cytoplasmic proteasome complex. Nonnative forms of some proteins that "escape" this surveillance system can accumulate and result in disease (for example neurodegenerative diseases like Alzheimers and Parkinson's disease.
Navigation
Return to Glycoprotein Synthesis and Function Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 3D: Glycoprotein Biosynthesis and Function

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.