Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
A: Lipid Structure
BIOCHEMISTRY - DR. JAKUBOWSKI
2/06/16
Learning Goals/Objectives for Chapter 1A: After class and this reading, students will be able to
|
1A1: Introduction to Lipids
Lipids are small biological molecules which are soluble in organic solvents, such as chloroform/methanol, and are sparingly soluble in aqueous solutions. They can be classified in a variety of ways. In one categorization, they can be divided into two majors classes, saponifiable and nonsaponifiable lipids, based on their reactivity with strong bases. Saponifiable lipids contain long chain carboxylic (of fatty) acids, that are linked to an alcoholic functional group through an ester linkage. These fatty acids are released on based catalyzed ester hydrolysis. The nonsaponifiable classes include the "fat-soluble" vitamins (A, E) and cholesterol. Lipids are often distinguished from another commonly used word, fats. Some define fats as lipids that contain fatty acids that are esterified to glycerol. I will use the lipid and fat synonymously.
Figure: Examples saponifiable and nonsaponifiable lipids
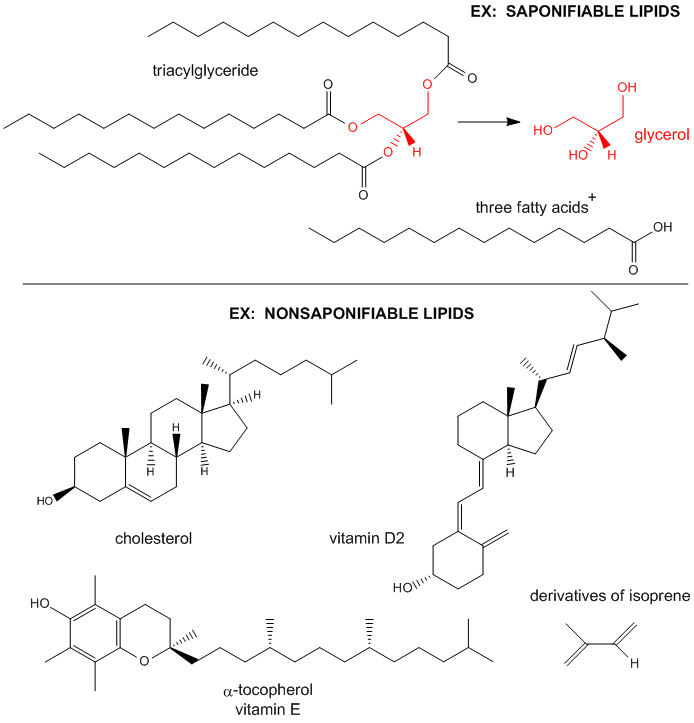
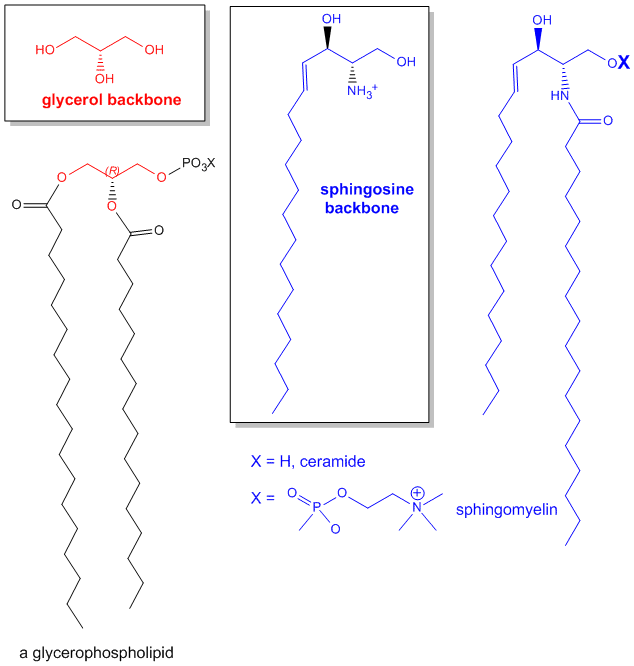
The major saponifiable lipids are triacylglycerides, glycerophospholipids, and the sphingolipids. The first two use glycerol as the backbone. Triacylglycerides have three fatty acids esterified to the three OHs on glycerol. Glycerophospholipids have two fatty acids esterified at carbons 1 and 2, and a phospho-X groups esterifed at C3. Sphingosine, the backbone for sphingolipids, has a long alkyl group connected at C1 and a free amine at C2, as a backbone. In sphingolipids, a fatty acid is attached through an amide link at C2, and a H or esterified phospho-X group is found at C3. A general diagrams showing the difference in these structures is shown below.
Figure: Comparison of lipids with glycerol and sphingosine as backbones
The simple classification of lipids based on their reactivity towards bases belies the complexity of possible lipid structures as over 1000 different lipids are found in eukaryotic cells. This complexity has led to the development of a comprehensive classification system for lipids. In this system, lipids are given a very detailed as well as all-encompassing definition: "hydrophobic or amphipathic small molecules that may originate entirely or in part by carbanion-based condensations of thioesters (fatty acyl, glycerolipids, glycerophospholipids, sphingolipds, saccharolipds and polyketides) and/or by carbocation-based condensations of isoprene units (prenol lipids and sterol lipids)."
Using this new nomenclature, lipids can be broken into eight different categories, as shown in the table below and at LIPID MAPS.
Table: Classification of Lipids Based on International Classification and Nomenclature Committee
| Category | Abbreviation | Example Structure |
| Fatty Acids | FA |
 |
| Glycerolipids | GL |
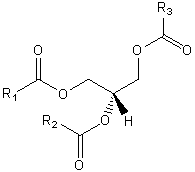 |
| Glycerophospholipids | GP |
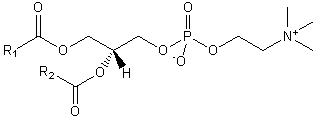 |
| Sphingolipids | SP |
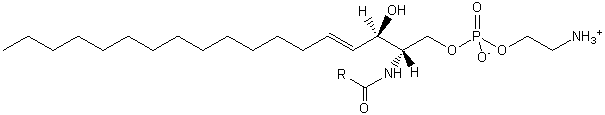 |
| Sterol Lipids | ST |
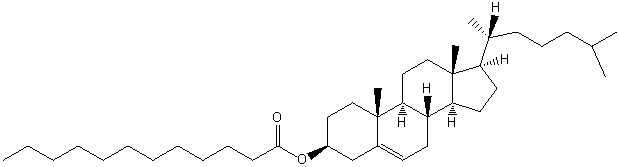 |
| Prenol Lipids | PR |
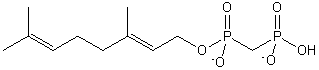 |
| Saccharolipids (contain sugar) |
SL |
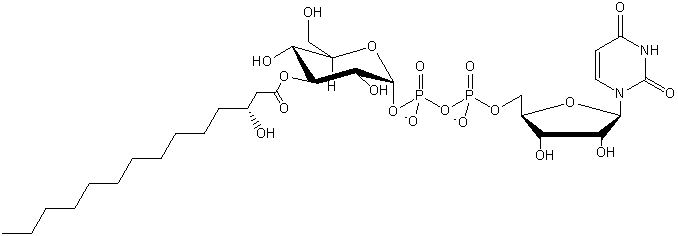 |
| Polyketides | PK |
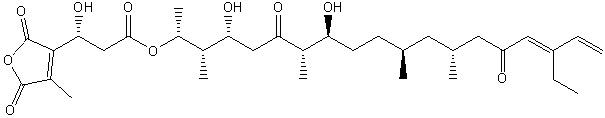 |
Properties of Lipids
The structure of lipids determines their function. For example, the very insoluble triacylglycerides are used as the predominant storage form of chemical energy in the body. In contrast to polysaccharides such as glycogen (a polymer of glucose), the Cs in the acyl-chains of the triacylglyceride are in a highly reduced state. The main source of energy to drive not only our bodies but also our society is obtained through oxidizing carbon-based molecules to carbon dioxide and water, in a reaction which is highly exergonic and exothermic. Sugars are already part way down the free energy spectrum since each carbon is partially oxidized. 9 kcal/mol can be derived from the complete oxidation of fats, in contrast to 4.5 kcal/mol from that of proteins or carbohydrates. In addition, glycogen is highly hydrated. For every 1 g of glycogen, 2 grams of water is H-bonded to it. Hence it would take 3 times more weight to store the equivalent amount of energy in carbohydrates as is stored in triacylglyceride, which are stored in anhydrous lipid "drops" within cells . The rest of this unit on lipids will focus not on triacylglycerides, whose main function is energy storage, but on fatty acids and phospholipids, and the structures they form in aqueous solution.
The structure of fatty acids and phospholipids show them to amphiphilic - i.e. they have both hydrophobic and hydrophilic domains. Fatty acids can be represented in "cartoon-form" as single chain amphiphiles with a circular polar head group and a single acyl non-polar tail extending from the head. Likewise, phospholipids can be shown as double chain amphiphiles. Even cholesterol can be represented this way, with its single OH group as the polar head, and the rigid 4 member rings as the hydrophobic “tail”. Even through there are a very large number of fatty acids which can be esterified to C1 and C2 of phospholipids and a variety of P-X groups at C3, making the phospholipids and fatty acids extremely heterogeneous groups of molecules, their role in biological structures can be understood to a first approximation by modeling them either as single or double chain amphiphiles. In addition, they, in contrast to carbohydrates, amino acids, and nucleotides, do not form covalent polymers. Hence we will start our studies of biological molecules with simple lipids (fatty acids, glycerophospholipids and sphingolipids) and then apply our understanding of lipids to the more complex systems of biological polymers. We will see that glycerophospholipids and sphingolipids are essential components of membrane structure. Cholesterol is also found in membranes and is a precursor of steroid hormones.
Navigation
Return to Chapter 1A: Lipid Structures Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 1A: Lipid Structure