Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
A: Lipid Structure
BIOCHEMISTRY - DR. JAKUBOWSKI
2/06/16
Learning Goals/Objectives for Chapter 1A: After class and this reading, students will be able to
|
1A2. Fatty Acids
Fatty acids can be saturated (contain no double bonds in the acyl chain), or unsaturated (with either one -monounsaturated - or multiple - polyunsaturated - double bond(s)) . The table below gives the names, in a variety of formats, of common fatty acids.
Table: Names and structures of the most common fatty acids
COMMON BIOLOGICAL SATURATED FATTY ACIDS
|
Symbol |
common name |
systematic name |
structure |
mp(C) |
|
12:0 |
Lauric acid |
dodecanoic acid |
CH3(CH2)10COOH |
44.2 |
|
14:0 |
Myristic acid |
tetradecanoic acid |
CH3(CH2)12COOH |
52 |
|
16:0 |
Palmitic acid |
Hexadecanoic acid |
CH3(CH2)14COOH |
63.1 |
|
18:0 |
Stearic acid |
Octadecanoic acid |
CH3(CH2)16COOH |
69.6 |
|
20:0 |
Arachidic aicd |
Eicosanoic acid |
CH3(CH2)18COOH |
75.4 |
COMMON BIOLOGICAL UNSATURATED FATTY ACIDS
|
Symbol |
common name |
systematic name |
structure |
mp(C) |
|
16:1Δ9 |
Palmitoleic acid |
Hexadecenoic acid |
CH3(CH2)5CH=CH-(CH2)7COOH |
-0.5 |
|
18:1Δ9 |
Oleic acid |
9-Octadecenoic acid |
CH3(CH2)7CH=CH-(CH2)7COOH |
13.4 |
|
18:2Δ9,12 |
Linoleic acid |
9,12 -Octadecadienoic acid |
CH3(CH2)4(CH=CHCH2)2(CH2)6COOH |
-9 |
|
18:3Δ9,12,15 |
α-Linolenic acid |
9,12,15 -Octadecatrienoic acid |
CH3CH2(CH=CHCH2)3(CH2)6COOH |
-17 |
|
20:4Δ5,8,11,14 |
arachidonic acid |
5,8,11,14- Eicosatetraenoic acid |
CH3(CH2)4(CH=CHCH2)4(CH2)2COOH |
-49 |
|
20:5Δ5,8,11,14,17 |
EPA |
5,8,11,14,17-Eicosapentaenoic- acid |
CH3CH2(CH=CHCH2)5(CH2)2COOH |
-54 |
|
22:6 Δ4,7,10,13,16,19 |
DHA |
Docosohexaenoic acid |
22:6w3 |
|
% FATTY ACIDS IN VARIOUS FATS
|
FAT |
<16:0 |
16:1 |
18:0 |
18:1 |
18:2 |
18:3 |
20:0 |
22:1 |
22:2 |
. |
|
Coco-nut |
87 |
. |
3 |
7 |
2 |
. |
. |
. |
. |
. |
|
Canola |
3 |
. |
|
11 |
13 |
10 |
. |
7 |
50 |
2 |
|
Olive Oil |
11 |
. |
4 |
71 |
11 |
1 |
. |
. |
. |
. |
|
Butter-fat |
50 |
4 |
12 |
26 |
4 |
1 |
2 |
. |
. |
. |
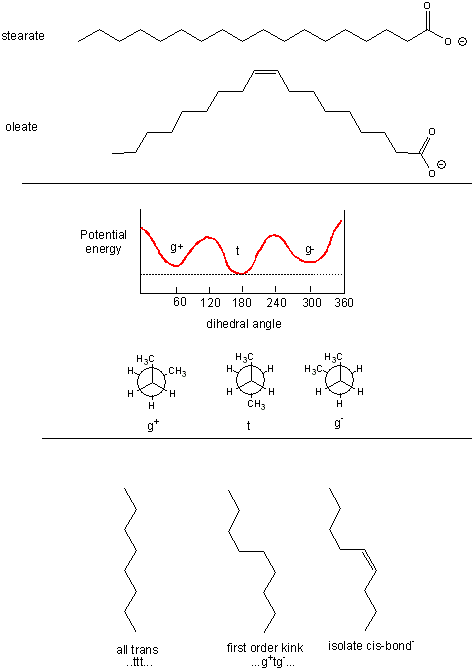
The figure below shows the relative conformations of saturated and unsaturated fatty acids, and in comparison, the conformations and potential energy graph for n-butane, which should provide insight into conformational changes in the nonpolar tail of fatty acids arising from rotation around C-C single bonds. We will explore this diagram a bit latter.
Figure: Conformations of fatty acids and n-butane
![]()
![]() Jmol:
conformations of ethane |
conformations of propane |
butane: the gauche conformation
Jmol:
conformations of ethane |
conformations of propane |
butane: the gauche conformation
Fatty acids can be named in many ways.
- symbolic name: given as x:y Δ a,b,c where x is the number of C’s in the chain, y is the number of double bonds, and a, b, and c are the positions of the start of the double bonds counting from C1 - the carboxyl C. Saturated fatty acids contain no C-C double bonds. Monounsaturated fatty acids contain 1 C=C while polyunsaturated fatty acids contain more than 1 C=C. Double bonds are usual cis.
- systematic name using IUPAC nomenclature. The systematic name gives the number of Cs (e.g. hexadecanoic acid for 16:0). If the fatty acid is unsaturated, the base name reflects the number of double bonds (e.g. octadecenoic acid for 18:1 Δ 9 and octadecatrienoic acid for 18:3Δ 9,12,15).
- common name: (e.g. oleic acid, which is found in high concentration in olive oil)
You should know the common name, systematic name, and symbolic representations for these saturated fatty:
- lauric acid, dodecanoic acid, 12:0
- palmitic acid, hexadecanoic acid, 16:0
- stearic acid, octadecanic acid, 18:0.
Learn the following unsaturated fatty acids -
- oleic acid, octadecenoic acid, 18:1 Δ 9
- linoleic acid, octadecadienoic acid, 18:2 Δ 9,12
- a-linolenic acid, octadecatrienoic acid, 18:3 Δ 9,12,15 (n-3)
- arachidonic acid, eicosatetraenoic acid, 20:4Δ 5,8,11,14 (n-6)
- eicosapentenoic acid (EPA), 20:5 Δ 5,8,11,14,17 (n-3) Note: sometimes written as eicosapentaenoic
- docosahexenoic acid (DHA) 22:6 Δ4,7,10,13,16,19 (n-3) Note: sometimes written as docosahexaenoic
There is an alternative to the symbolic representation of fatty acids, in which the Cs are numbered from the distal end (the n or w end) of the acyl chain (the opposite end from the carboxyl group). Hence 18:3 Δ 9,12,15 could be written as 18:3 (w -3) or 18:3 (n -3) where the terminal C is numbered one and the first double bond starts at C3. Arachidonic acid is an (w -6) fatty acid while docosahexaenoic acid is an (w -3) fatty acid.
Note that all naturally occurring double bonds are cis (E), with a methylene spacer between double bonds - i.e. the double bonds are not conjugated. For saturated fatty acids, the melting point increases with C chain length, owing to increased likelihood of van der Waals (London or induced dipole) interactions between the overlapping and packed chains. Within chains of the same number of Cs, melting point decreases with increasing number of double bonds, owing to the kinking of the acyl chains, followed by decreased packing and reduced intermolecular forces (IMFs). Fatty acid composition differs in different organisms:
- animals have 5-7% of fatty acids with 20-22 carbons, while fish have 25-30%
- animals have <1% of their fatty acids with 5-6 double bonds, while plants have 5-6% and fish 15-30%
Many studies support the claim the diets high in fish that contain abundant n-3 fatty acids, in particular EPA and DHA, reduce inflammation and cardiovascular disease. n-3 fatty acids are abundant in high oil fish (salmon, tuna, sardines), and lower in cod, flounder, snapper, shark, and tilapia.
The most common polyunsaturated fats (PUFAs) in our diet are the n-3 and n-6 classes. Most abundant in the n-6 class in plant food is linoleic acid (18:2n-6, or 18:2Δ9,12), while linolenic acid (18:3n-3 or 18:3Δ9,12,15) is the most abundant in the n-3 class. These fatty acids are essential in that they are biological precursors for other PUFAs. Specifically,
- linoleic acid (18:2 n-6, or 18:2Δ9,12) is a biosynthetic precursor of arachidonic acid (20:4n-6 or 20:4Δ5,8,11,14)
- linolenic acid (18:3n-3, or 18:3Δ9,12,15) is a biosynthetic precursor of eicosapentaenoic acid (EPA, 20:5n-3 or 20:5Δ5,8,11,14,17) and to a much smaller extent, docosahexaenoic acid (DHA, 22:6n-3 or 22:6Δ4,7,10,13,16,19).
These essential precursor fatty acids are substrates for intracelluar enzymes such as elongases, desaturases, and beta-oxidation type enzymes in the endoplasmic reticulum and another organelle, the peroxisome (involved in oxidative metabolism of straight chain and branched fatty acids, peroxide metabolism, and cholesterol/bile salt synthesis). Animals fed diets high in plant 18:2(n-6) fats accumulate 20:4(n-6) fatty acids in their tissues while those fed diets high in plant 18:3(n-3) accumulate 22:6(n-3). Animals fed diets high in fish oils accumulate 20:5 (EPA) and 22:6 (DHA) at the expense of 20:4(n-6).
Recent work has suggested that contrary to images of early hominids as hunters and scavengers of meat, human brain development might have required the consumption of fish which is highly enriched in arachidonic and docosahexaenoic acids. A large percent of the brain consists of lipids, which are highly enriched in these two fatty acids. These acids are necessary for the proper development of the human brain and in adults, deficiencies in these might contribute to cognitive disorders like ADHD, dementia, and dyslexia. These fatty acids are essential in the diet, and probably could not have been derived in high enough amounts from the eating of brains of other animals. The mechanism for the protective effects of n-3 fatty acids in health will be explored later in the course when we discuss prostaglandins synthesis and signal transduction.
Saturated fatty acids chains can exist in many conformations resulting from free rotation around the C-C bonds of the acyl chains. A quick review of the conformations of n-butane shows that the energetically most favorable conformation is one in which the two CH3 groups attached to the 2 methylene C’s (C2 and C3) are trans to each other, which results in decreased steric strain. Looking at a Neuman projection of n-butane shows the dihedral or torsional angle of this trans conformation to be 180 degrees. When the dihedral angle is 0 degrees, the two terminal CH3 groups are syn to each other, which is the conformation of highest energy. When the angle is 60 (gauche+) or 300 (gauche-) degrees, a higher, local minimum is observed in the energy profile. At a given temperature and moment, a population of molecules of butane would consist of some in the g+ and g- state, with most in the t state. The same applies to fatty acids. To increase the number of chains with g+tg- conformations, for example, the temperature of the system can be increased.
Navigation
Return to Chapter 1A: Lipid Structures Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 1A: Lipid Structure