Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
A: Lipid Structure
BIOCHEMISTRY - DR. JAKUBOWSKI
2/06/16
Learning Goals/Objectives for Chapter 1A: After class and this reading, students will be able to
|
1A3. Glycerophospholipid and Sphingolipids
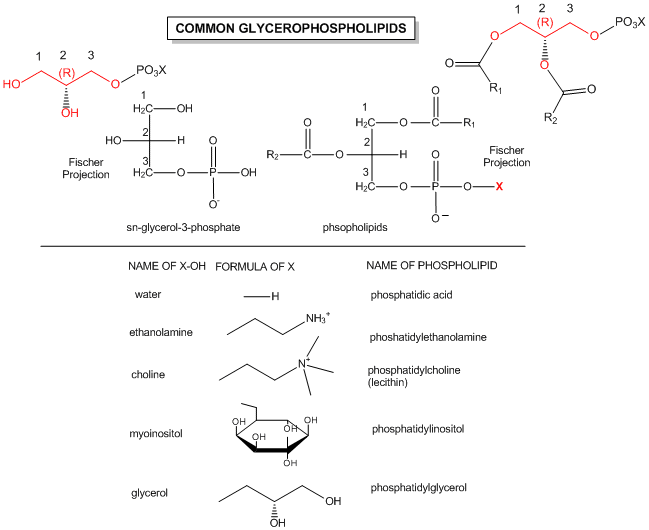
The generic structures of glycerophospholipid is show below, along with the most common glycerophospholipids. Learn the structures of phosphatic acid (PA), phosphatidyethanolamine (PE), phosphatidylcholine (PC) which is often called lechitin, phosphatidylserine (PS) which is often called cephalin, and sphingomylein (shown in an earlier figure).
Figure: Structures of common phospholipids
![]() Updated
di-18:0 PC
Jmol14 (Java) |
JSMol (HTML5)
Updated
di-18:0 PC
Jmol14 (Java) |
JSMol (HTML5)
![]() Updated
Triacylglyceride
Jmol14 (Java) |
JSMol (HTML5)
Updated
Triacylglyceride
Jmol14 (Java) |
JSMol (HTML5)
Triacylglyceride/Phospholipid Stereochemistry
Glycerol is an achiral molecule, since C2 has two identical substituents, -CH2OH. Glycerol in the body can be chemically converted to triacylglycerides and phospholipids (PL) which are chiral, and which exist in one enantiomeric form. How can this be possible if the two CH2OH groups on glycerol are identical? It turns out that even though these groups are stereochemically equivalent, we can differentiate them as follows. Orient glycerol with the OH on C2 pointing to the left. Then replace the OH of C1 with OD, where D is deuterium. Now the two alcohol substituents on C1 and C3 are not identical and the resulting molecule is chiral. By rotating the molecule such that the H on C2 points to the back, and assigning priorities to the other substituents on C2 as follows: OH =1, DOCH2 =2, and CH2OH = 3, it can be seen that the resulting molecule is in the S configuration. Hence we say that C1 is the proS carbon. Likewise, if we replaced the OH on C3 with OD, we will form the R enantiomer. Hence C3 is the proR carbon. This shows that in reality we can differentiate between the two identical CH2OH substituents. We say that glycerol is not chiral, but prochiral. (Think of this as glycerol has the potential to become chiral by modifying one of two identical substituents.)
Figure: Glycerol - A prochiral molecule
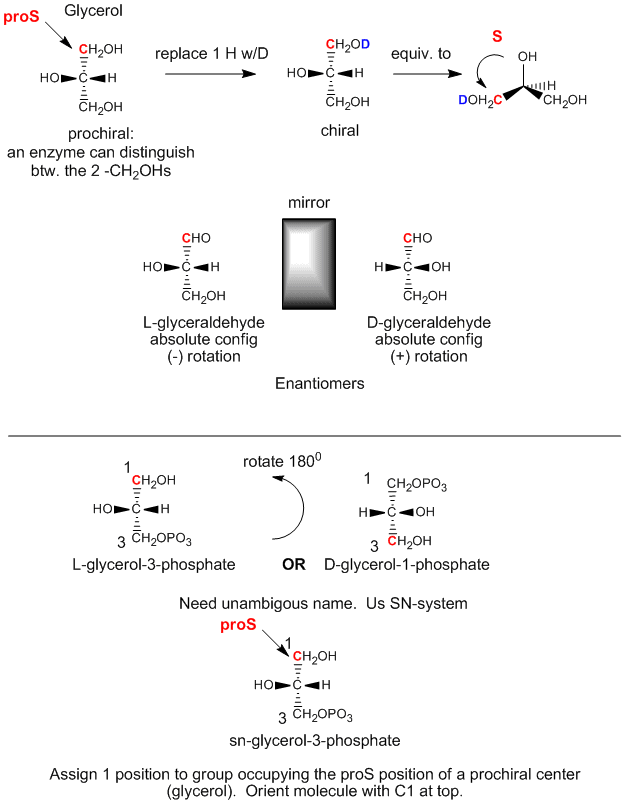
We can relate the configuation of glycerol above, (when OH on C2 is pointing to the left) to the absolute configuration of L-glyceraldehyde, a simple sugar (a polyhydroxyaldehyde or ketone), another 3C glycerol derivative. This molecule is chiral with the OH on C2 (the only chiral carbon) pointing to the left. It is easy to remember that any L sugar has the OH on the last chiral carbon pointing to the left. The enantiomer (mirror image isomer) of L-glyceraldehyde is D-glyeraldehyde, in which the OH on C2 points to the right. Biochemists use L and D for lipid, sugar, and amino acid stereochemistry, instead of the R,S nomenclature you used in organic chemistry. The stereochemical designation of all the sugars, amino acids, and glycerolipids can be determined from the absolute configuration of L- and D-glyceraldehyde.
The first step in the in vivo (in the body) synthesis of chiral derivatives from the achiral glycerol involves the phosphorylation of the OH on C3 by ATP (a phosphoanhydride similar in structure to acetic anhydride, an excellent acetylating agent) to produce the chiral molecule glycerol phosphate. Based on the absolute configuration of L-glyceraldehyde, and using this to draw glycerol (with the OH on C2 pointing to the left), we can see that the phosphorylated molecule can be named L-glycerol-3-phosphate. However, by rotating this molecule 180 degrees, without changing the stereochemistry of the molecule, we don't change the molecule at all, but using the D/L nomenclature above, we would name the rotated molecule as D-glycerol-1-phosphate. We can’t give the same molecule two different names. Hence biochemists have developed the stereospecific numbering system (sn), which assigns the 1-position of a prochiral molecule to the group occupying the proS position. Using this nomenclature, we can see that the chiral molecule described above, glycerol-phosphate, can be unambiguously named as sn-glycerol-3-phosphate. The hydroxyl substituent on the proR carbon was phosphorylated.
Figure: The biological synthesis of triacylglycerides and phosphatidic acid from prochiral glycerol
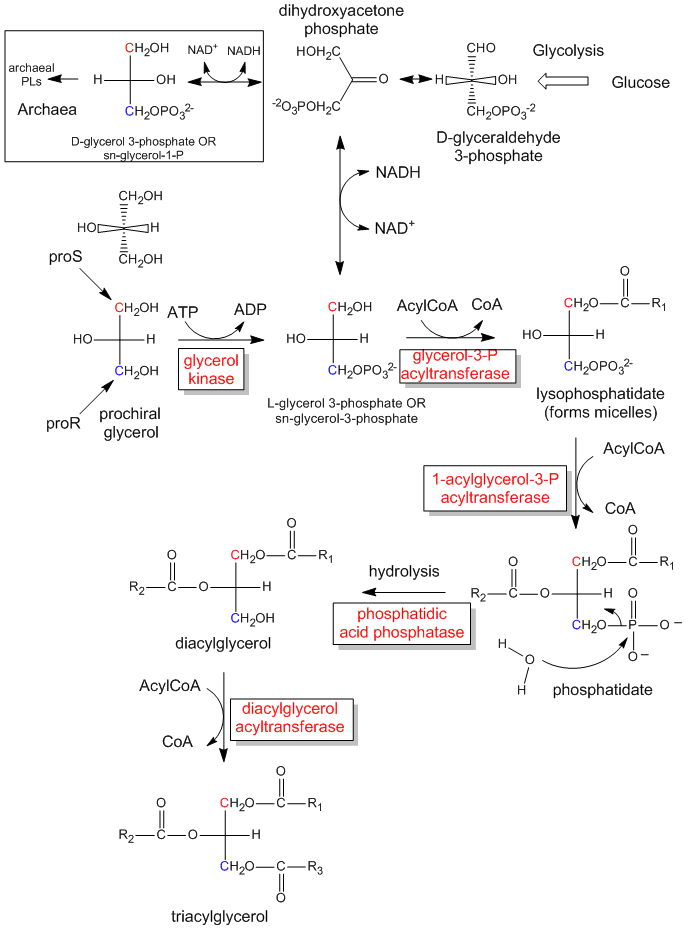
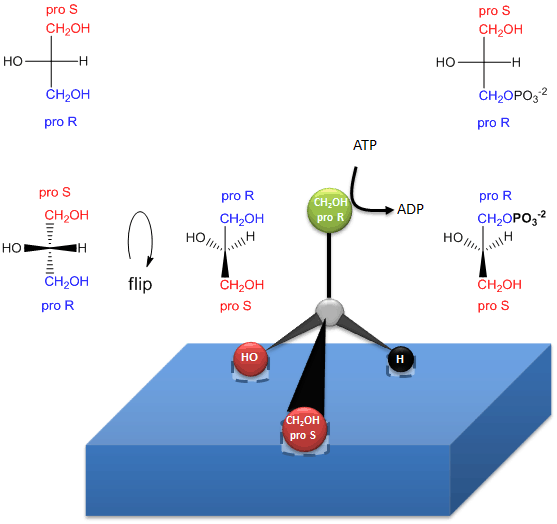
The enzymatic phosphorylation of prochiral glycerol on OH of the proR carbon to form sn-glycerol-3-phosphate is illustrated in the link below. As we were able to differentiate the 2 identical CH2OH substitutents as containing either the proS or proR carbons, so can the enzyme. The enzyme can differentiate identical substituents on a prochiral molecule if the prochiral molecule interacts with the enzyme at three points. Another example of a prochiral reactants/enzyme system involves the oxidation of the prochiral molecule ethanol by the enzyme alcohol dehydrogenase, in which only the proR H of the 2 H’s on C2 is removed. (We will discuss this later.)
Figure: How an enzyme (glycerol kinase)
transfers a PO4 from ATP to the proR CH2OH of glycerol
on
formation of chiral triacylglycerols and phosphatidic acid.
Navigation
Return to Chapter 1A: Lipid Structures Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 1A: Lipid Structure