Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 1 - LIPID STRUCTURE
A: Lipid Structure
BIOCHEMISTRY - DR. JAKUBOWSKI
2/06/16
Learning Goals/Objectives for Chapter 1A: After class and this reading, students will be able to
|
1A4. Lipids in Archea, Prokaryotes and Eukaryotes
Do all organisms use the same lipid building blocks to construct bilayers? It turns out they don't. Life can be divided into three separate domains, Bacteria, Archea, and Eukaryota. Studies of sequence similarities of the ribosomal RNA genes from the DNA of these cells show that archea and eukaryota are more closely related than bacteria (also called prokaryotes). Yet there are many similarities between archea and bacteria. Both bacteria and archea are single-celled organisms without nuclei and internal organelles. In the past archea were thought to belong to the prokaryotes. Yet they differ significantly in genetic structure and in their metabolic pathways.
Figure: Phylogenetic Tree of Life
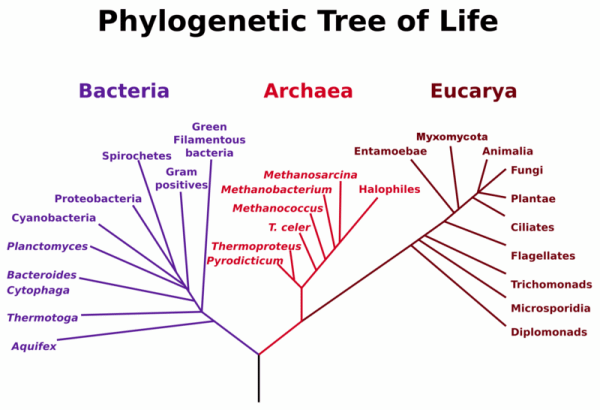
This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license
Archea often have very unique chemistries. Members of this domain can use not only carbohydrates and fats as sources of energy, but they can also use inorganic species such as ammonium, hydrogen, and metal ions as well as organic molecules such as methane. Some (methanogens) actually make methane. Archea were once thought to be found only in extreme environments (hence they were also called extremophiles), but in actuality they inhabit many environmental niches, including the oceans and soil. Since many do live in extreme environments, you would expect them to have evolved to synthesize stable, structural molecules. Archea use phospholipids in the membrane bilayers, but the lipids differ in three very important ways. Instead of fatty acid chains, they use isoprenoid chains as the nonpolar chains. Instead of using an ester link, the isoprenoids are covalently attached to the glycerol backbone with an ether link, which is obviously more stable than an ester bond used in the phospholipids discussed above. Finally, the stereochemistry of the phospholipids is based on sn-glycerol-1-phosphate and not sn-glycerol-3-phosphate.
Navigation
Return to Chapter 1A: Lipid Structures Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 1A: Lipid Structure