Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8: OXIDATIVE-PHOSPHORYLATION
A: THE CHEMISTRY OF DIOXYGEN
BIOCHEMISTRY - DR. JAKUBOWSKI
04/14/16
|
Learning Goals/Objectives for Chapter 8A: After class and this reading, students will be able to
|
A10. Hypoxia
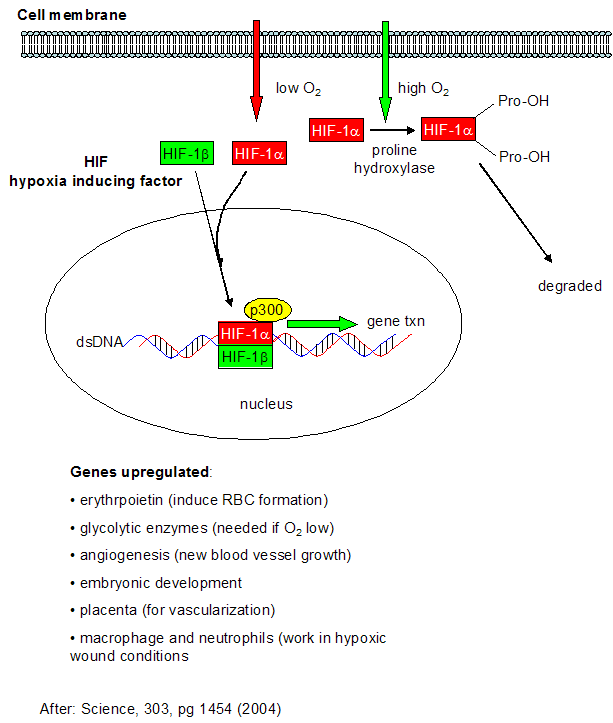
We have spend much time studying how the body deals with and utilizes the toxic byproducts of dioxygen reduction. What happens when the body doesn't get enough dioxygen - a condition call anoxia (no dioxygen) or hypoxia (too little dioxygen)? This might occur in muscles undergoing vigorous exercise, and in the brain and heart when clots occlude blood flow to these organs (as occurs in most strokes and heart attacks). Under low dioxygen concentrations, a family of protein transcription factors called hypoxia-inducible factor (HIF) become activated. The functional proteins appears to be a dimer of HIF-α and HIF-β. In contrast to the concentration of the beta form, the activity of HIF-α is increased under low dioxygen concentrations. HIF-α concentration is regulated not at the transcriptional level, but through proteolysis of the protein. In the presence of abundant dioxygen, HIF-α is hydroxylated at two Pro residues by the enzyme prolyl hydroxlase. This post-translational modification targets the protein for proteolysis (through ubiquitination by the VHL protein and subsequent cleavage by the proteasome). A second independent pathway in rapidly growing tissue leads to increased expression of HIF-α even in the presence of dioxygen. This could be beneficial to cells since rapidly growing tissue, especially tumor cells, might be expected to experience low oxygen conditions.
Figure: Cell response to hypoxia
Navigation
Return to Chapter 8A: The Chemistry of Dioxygen Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8A: The Chemistry of Dioxygen

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.