Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8: OXIDATIVE-PHOSPHORYLATION
A: THE CHEMISTRY OF DIOXYGEN
BIOCHEMISTRY - DR. JAKUBOWSKI
04/14/16
|
Learning Goals/Objectives for Chapter 8A: After class and this reading, students will be able to
|
A3. The Reactions of Dioxygen and its Reduction Products
1. Triplet O2 - Ground State:
Figure: Triplet O2 - Ground State
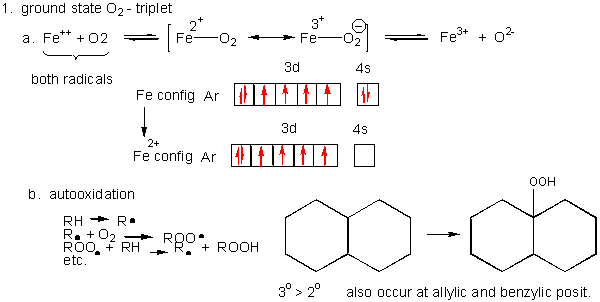
- Metals ions - Metal ions are radicals
themselves, so can react with dioxygen. Ex:
Fe2+ + O2 <--> [ Fe2+-- O2 <--> Fe3+-- O2-.] <--> Fe3+ + O2-. (superoxide) - Autoxidation of organic molecules to
produce peroxides. In this free radical reaction, several reactions
occur, including
RH ---> R. (Initiation)
R. + O2 ---> ROO. (Propagation)
ROO. + RH ---> R. + ROOH (Propagation)
R. + R. ---> R--R (Termination)
ROO. + ROO. ---> ROOR + O2 (Termination)
ROO. + R. ---> ROOR (Termination)
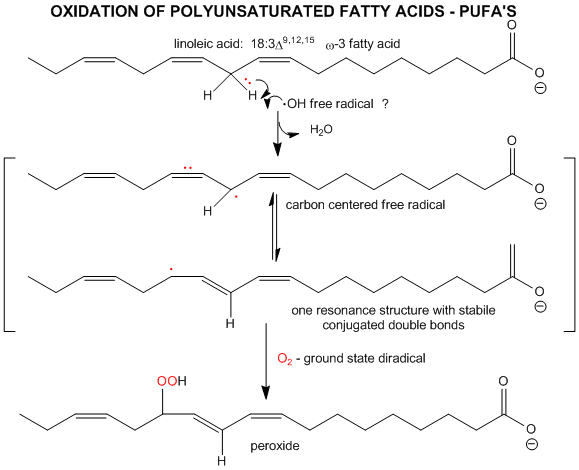
The initiation step above occurs mostly at C atoms which can produce the most stable free radicals (allylic, benzylic position, and 3o > 2o >> 10 carbons). Hence unsaturated fatty acids are extra reactive at the methylene C that separate the double bonds.
Figure: unsaturated fatty acids are extra reactive at the methylene C that separate the double bonds
2. Single O2 - Excited State.
Figure: Single O2 - Excited State
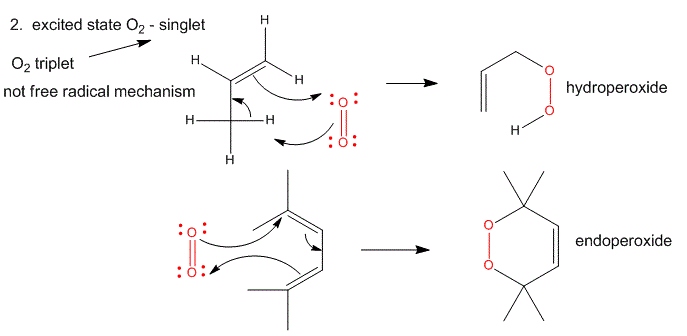
It can be made from triplet oxygen by photoexcitation. Alternatively, it can be made from triplet oxygen through collision with an excited molecule which relaxes to the ground state after a radiationless transfer of energy to triplet oxygen to form reactive singlet oxygen. (This later process accounts for photobleaching of colored clothes when the conjugated dye molecules absorb UV and Vis light, relax by transferring energy to triplet oxygen to form singlet oxygen, which then chemically reacts with the conjugated double bonds in the dye. )
- Alkenes react with oxygen to form hydroperoxides, potentially through a epoxide intermediate
- Dienes reacts with oxygen in a Diels-Alder like reaction to form endoperoxides
3. Superoxide
Figure: Superoxide
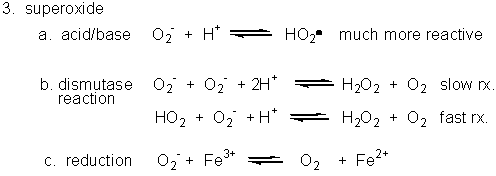
- Dismuation: O2-. + O2-. ---> H2O2 + O2
- Acid/Base: HO2. ----> O2-. + H+ (pKa = 4.8)
- With metal ions: Fe3+ (as in heme) + O2-. ---> O 2 + Fe2+
4. Peroxide
Figure: Peroxide
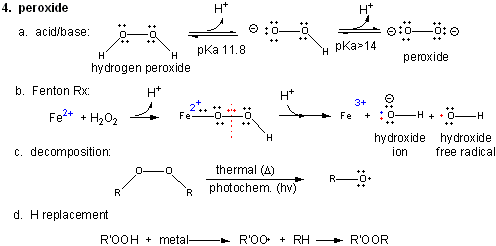
In contrast to dioxygen which contains multiple bonds between the O atoms, peroxide has only one bond. In fact, it is quite weak and requires only 38 kcal/mol to break it. Remember, bonds can be broken in a heterolytic way (both electrons in a bond go to one of the atoms, or in a homolytic fashion, in which the one electron goes to each atom.
- Acid/Base: H2O2 ---> HO2- ---> O22- (pKa1 = 11.8; pKa2 > 14)
- Reaction with Fe2+ - The Fenton Reaction:
(similar to reaction of triplet O2 with Fe2+ above)
Fe2+ + OOH- <--> Fe2+-- OOH <--> Fe2+-- O + OH- <--> Fe3+-- O . <--> Fe3+ + OH. (last step a proton is added). In this reaction, a homolytic cleavage of the O--O bond occurs generating OH- and the hydroxy free radical, OH., which will react with any molecule it encounters. - thermal or photochemical homolytic cleavage of peroxide. This forms alkoxide free radicals which reacts like the hydroxy free radical.
- Reactions with alkyl groups in the
presence of metal ions such as Cu, Co, or Mn:
RH + R'OOH ----> ROOR'
5. Hydroxy free radical:
Figure: Hydroxy free radical
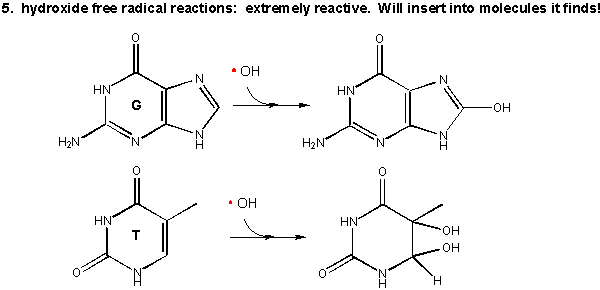
As mentioned above this species is extremely reactive. It will react with any molecule it encounters and does so immediately. It can abstract a H atom leaving another free radical. For example, the hydroxy free radical could extract a hydrogen atom from a polyunsaturated fatty acid to from a carbon-centered radical. A particularly nasty reaction is the insertion of the hydroxy radical into bases in DNA, as shown in the diagram.
Navigation
Return to Chapter 8A: The Chemistry of Dioxygen Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8A: The Chemistry of Dioxygen

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.