Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8: OXIDATIVE-PHOSPHORYLATION
A: THE CHEMISTRY OF DIOXYGEN
BIOCHEMISTRY - DR. JAKUBOWSKI
04/14/16
|
Learning Goals/Objectives for Chapter 8A: After class and this reading, students will be able to
|
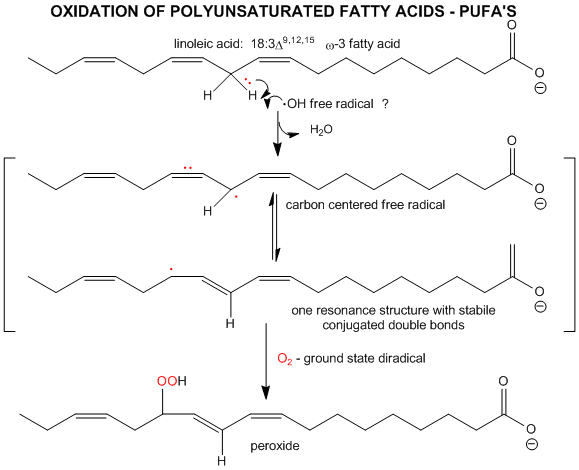
A5. Oxidative Modification of Lipids:
Figure: Oxidative Modification of Lipids
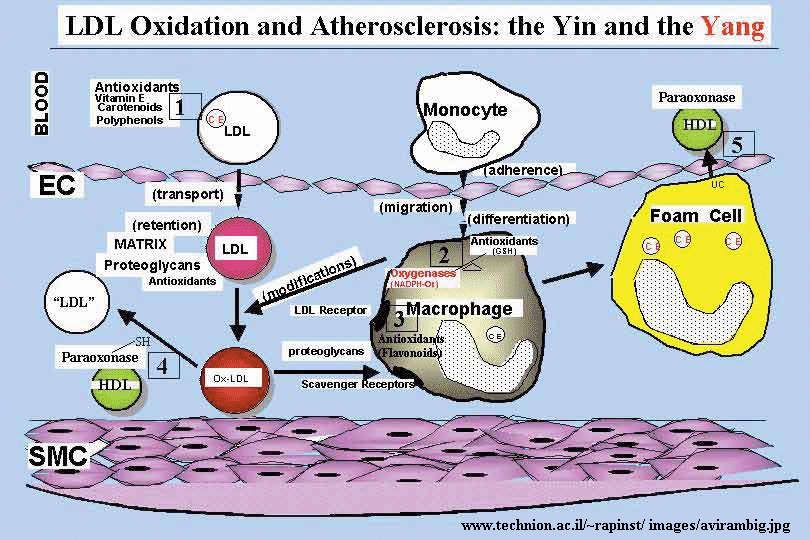
The initial stages of cardiovascular disease appear to involve the development of fatty acid streaks under the artery walls. Macrophages, an immune cell, have receptors which appear to recognize oxidized lipoproteins in the blood, which they take-up. The cells then become fat-containing foam cells which form the streaks. Oxidation of fatty acids in lipoproteins (possibly by ozone) could produce lipid peroxides and to protein oxidation in lipoproteins. Cortical neurons from fetal Down's Syndrome patients show 3-4 times levels of intracellular reactive O2 species and increased levels of lipid peroxidation compared to control neurons. This damage is prevented by treatment of the neurons in culture with free radical scavengers or catalase.
Figure: oxidized LDL uptake
A recent study of peroxiredoxins by Neumann et al showed the importance of these gene products in mice. Peroxidredoxins (which catalyze the conversion of a peroxides and thioredoxin into water and oxidized thioredoxin) are small proteins with an active site cysteine and are found in most organisms. Transcription of the mammalian peroxiredoxin 1 gene is activated by oxidative stress. They inactivated the gene which produced a mouse that could reproduce and appeared vital, but which had a shortened lifespan. These mice developed severe hemolytic anemia and several types of cancers. High levels of reactive oxygen species and resulting increased levels of oxidized proteins were found in red blood cells of the knockout mice with anemia. High levels of 8-oxoguanine, resulting from oxidative damage to DNA, were found in tumor cells.
Navigation
Return to Chapter 8A: The Chemistry of Dioxygen Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8A: The Chemistry of Dioxygen

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.