Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
D: THE LIGHT REACTION OF PHOTOSYNTHESIS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8D: After class and this reading, students will be able to
|
D3. The Light Reactions of Photosynthesis
Photoexcitation of the non-reaction center chlorophyll turns that molecule into a good reducing agent, which transfers its electron to the nearest excited state level of the reaction center chlorophyll. If you count both step together, the non-reaction center chlorophyll gets "photooxidized", in the process producing the "strong" oxidizing agent which is the positively charged chlorophyll derivative. The extra electron passed onto the second molecule will eventually be passed on to NADP+ to produce NADPH. The light reaction of photosynthesis in green plants is shown below. In this process, in a scheme that is reminiscent of electron transport in mitochondria, water is oxidized by photosystem II. Electrons from water are moved through PSII to a mobile, hydrophobic molecule, plastaquinone (PQ) to form its reduced form, PQH2. PSII is a complicated structure with many polypeptide chains, lots of chlorophylls, and Mn, Ca, and Fe ions. A Mn cluster, called the oxygen evolving complex, OEC, is directly involved in the oxidation of wate. Two key homologous 32 KD protein subunits, D1 and D2, in PSII are transmembrane proteins and are at the heart of the PSII complex. Another photosystem, PS1, is also found further "downstream" in the electron transport pathway. It takes electrons from another reduced mobile carrier of electrons, plastocyanin (PCred) to ferredoxin, which becomes a strong reducing agent. Ferrodoxin is a protein with an Fe-S cluster (Fe-S-Fe-S in a 4 membered ring, with 2 additions Cys residues coordinating each Fe). It ultimately passes its electrons along to NADP+ to form NADPH. A summary of the light reaction in plants and standard reduction potentials of the participants, are shown below. Note that many of the complexes produce a transmembrane proton gradient. In contrast to mitochondria, the lumen (as compared to the mitochondrial matrix) becomes more acidic that the other stroma. Protons then can move down a concentration gradient through the C0C1ATPase to produce ATP required for reductive biosythesis of glucose.
Figure: THE LIGHT REACTIONS OF PHOTOSYNTHESIS
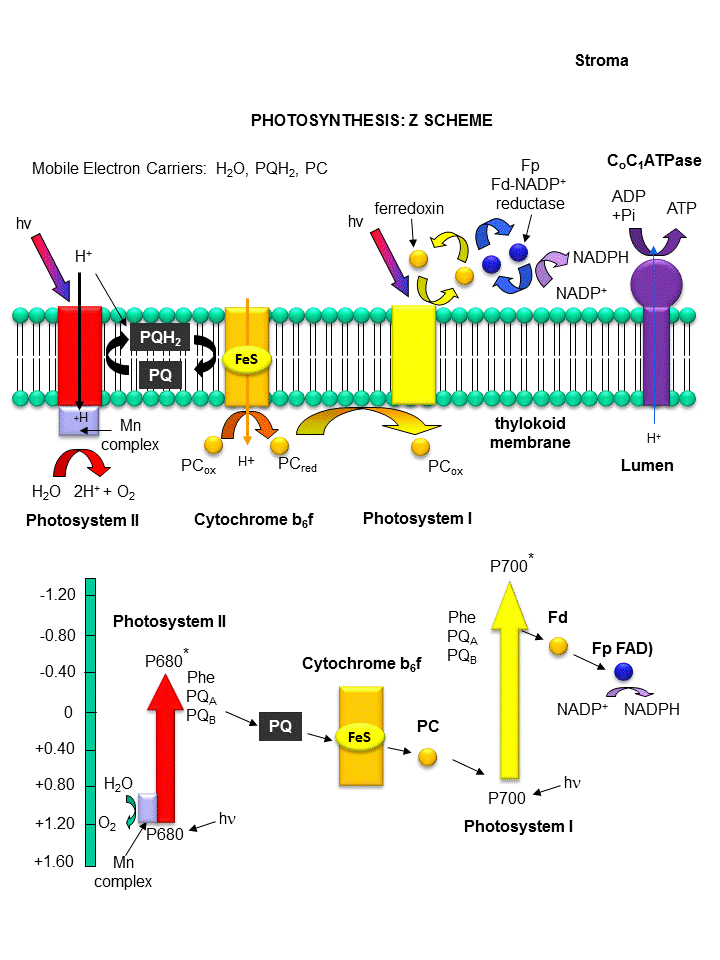
Figure: Detailed View of Light Reaction of Photosynthesis (reprinted with permission from Kanehisa Laboratories and the KEGG project: www.kegg.org )
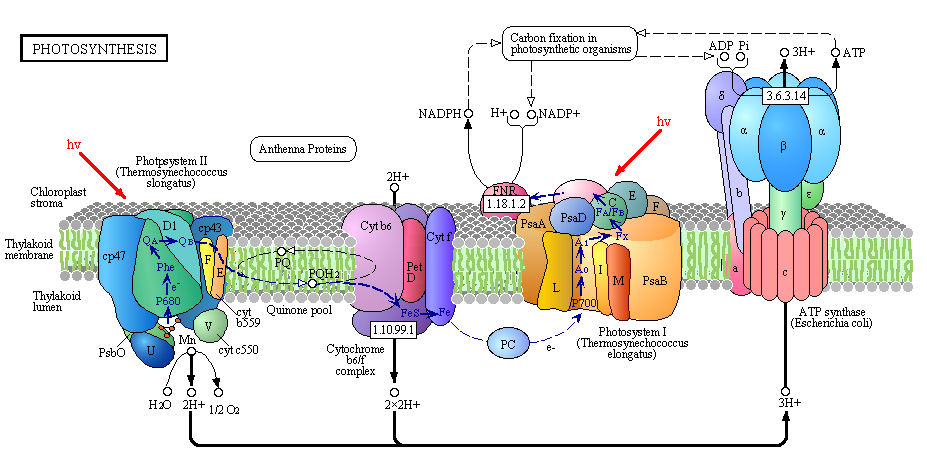
Boxed number represent Enzyme Commission Number. Original KEGG Map with imbedded links.
Navigation
Return to Chapter 8D: The Light Reactions of Photosynthesis Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8D: The LIght Reaction of Photosynthesis

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.