Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
D: THE LIGHT REACTION OF PHOTOSYNTHESIS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8D: After class and this reading, students will be able to
|
D5. The Oxygen Evolving Complex (OEC)
The crystal structure of PS2 from T. vulcanus has significantly improved our understanding of the OEC and electron flow on water oxidation.Because of its similarity to other pathways you have studied, we will concentrate on developing an understanding of the amazing Photosystem II from Thermosynechococcus vulcanus, a cyanobacteria (19 subunits with 35 chlorophylls, two phenophtyins, 11 beta cartotenes, 2 plastoquinones, 2 heme irons, 1 non heme iron, 4 Mn ions, 3-4 Ca ions, 3 Cl ions, 1 carbonate ion, and around 2800 water molecules).
Nature has appeared to evolve a single gene for the part of PSII that binds an inorganic cluster involved in oxygen formation. The cluster is called the Oxygen Evolving Complex (OEC) and is bound to a central protein in PS2. The cluster, Mn4CaO5, appears identical in all photosynthetic organisms, and is shown below. Researchers were surprise to find that the Ca ion was an integral part of the basic geometric “framework” of the OEC instead of a Mn which was found to be “dangling” from the basic geometric framework.
Figure: OEC structure from T. vulcanus
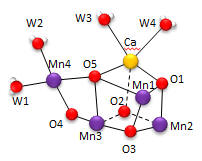
The shape outlined by O5-Ca-O1-Mn1 and Mn3-O2-Mn2-O3 is similar to what organic molecule? Describe how every two adjacent Mn are linked. Describe the coordination of Ca. Four waters (W1-W4) are coordinated to the OEC. No others were found in the region. What role may some of the waters have in the complex?
The amino acid side chains that interact with the OEC are shown below. The coordination number for all the Mn ions (including interacting waters) is identical. What is it for Mn1? For Ca? (Note that the Ala 344 is at the carboxy terminus of one of the protein subunits.)
Figure: OEC and surrounding amino acids from T. vulcanus
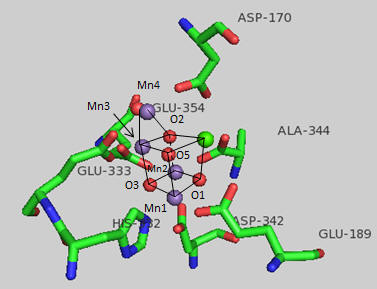
The OEC can be thought of as a distorted chair with a Mn3-O4-Mn4-O5 back. Bonds to O5 are longer than the other bonds. What might that imply about the strength of the bonds compared to the other metal-oxygen bonds? What is the expected charge of the oxo ligands in the OEC? If the metal-O5 bond lengths are longer than the others, what might that imply about the charge on O5? Would it be consistent with a ligand other than an oxo ligand? What might it be?
Which waters are most proximal to O5? Ultimately an O-O bond must form between two waters. Which are the most likely candidates based on the structure above? Which ion, Ca or Mn, would most likely be involved in electron transfer to water oxygens? Write the electron configuration for both ion. Standard reduction potentials for Mn are shown below. What is the oxidation number of Mn in each compound. Which oxidation state might be sufficient for the oxidation of H2O in the OEC?
- Mn2+ (aq) + 2 e-→ Mn (s) -1.185
- MnO4- (aq) + 2 H2O (l) + 3 e- → MnO2 (s) + 4 OH- 0.595
- MnO2(s) + 4H+ + e- → Mn3+ + 2H2O +0.95
- MnO2(s) + 4H+ + 2e- → Mn2+ + 2H2O +1.23
- MnO4- (aq) + 8 H+ (aq) + 5 e- → Mn2+ (aq) + 4 H2O (l) 1.507
- MnO4- (aq) + 4 H+ (aq) + 3 e- → MnO2 (s) + 2 H2O (l) 1.679
- HMnO4- + 3H+ + 2e- → MnO2(s) + 2H2O +2.09
- O2(g) + 4H+ + 4e- → 2H2O +1.229
The first structure of OEC above shows the two di-m-oxybridges that connect Mn4 to Mn1 and Mn3. This might give us clues as to the mechanism of water oxidation by the complex. To form O2, two water molecules are required as substrates. Presumably they bind as waters between adjacent Mns . What reaction intermediates may be bound to the Mn as water converts to O2? How many electrons are required for the complete oxidation?
Navigation
Return to Chapter 8D: The Light Reactions of Photosynthesis Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8D: The LIght Reaction of Photosynthesis

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.