Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
E: NITROGENASE - A REDUCTIVE USE OF METAL CENTERS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8D:
|
E1. Nitrogenase: An Introduction
Beauty is in the eye of the beholder.
As the domain biochemistry covers the entire biological world, the extent of coverage of a given topic in textbooks can depend, in part, on the interest and experiences of the author(s) presenting the material. Is relevance a metric that should determine coverage? If so, books focused on human or medical biochemistry would surely omit photosynthesis. If topics are selected based on their importance for life, then photosynthesis must surely be covered. If so, then nitrogenase must also be included. If the degree of chemical difficulty for a chemical reaction and the amazing eloquence of the evolved biochemistry solution is considered, then both photosynthesis and nitrogen fixation must be presented. Even though nitrogen fixation is a reductive reaction, it shares strong similarities with the oxygen evolving complex of photosynthesis. They catalyze enormously important redox reactions that involve an abundant atmospheric gas using a very complicated and unique inorganic metallic cofactor that evolution has selected as uniquely suited for the job.
Every first year student of chemistry can draw the Lewis structure of dinitrogen, N2, which contains a triple bond and a lone pair on each nitrogen. If Lewis structures speak to them, they should be able to state that the triple bond makes N2 extraordinarily stable, thus explaining why we can breathe an atmosphere containing 80% N2 and not die. If they have taken biology, they are also aware that very few biological organisms can utilize N2 as a substrate, as this require breaking bonds between the nitrogen atoms, a chemical process reserved for nitrogen “fixing” bacteria found in rhizomes of certain plants. Lastly, they probably memorized that high pressure and temperature, in a process called the Haber-Bosch process, is used react N2 and H2 to form ammonia, NH3. As with any scientific advance, the Haber-Bosch process has brought both harm (its used for explosive weapons) and good (fertilizers). This process now fixes enough N2 in the form of fertilizers to support half of the world’s population, with nitrogenase supporting the rest. Effort is being devoted to genetically modify plants to make their own nitrogenase, eliminating the need for fertilizers but perhaps creating unforeseen problems of its own.
You might be surprised to find out that at room temperature the equilibrium constant favors ammonia formation, hence DG0 < 0. The reaction is favored enthalpically as it is exothermic at room temperature. It is disfavored entropically as should be evident from the balanced equation below: N2(g) + 3H2(g) → 2 NH3(g)
If the reaction is favored thermodynamically at room temperature, why doesn’t it proceed? This story sounds familiar as this same descriptor applies to oxidation of organic molecules with dioxygen. There we showed using MO theory that the reaction is kinetically slow. Same with NH3 formation. A superficial way to see this is that we must break bonds in the stable N2 to start the reaction, leading to a high activation energy, making the reaction kinetics sluggish.
One could jump start the reaction by raising the temperature, but that would slow an exothermic reaction. The Keq (or KD) and DG0 are obviously functions of temperature and for this reaction, and the reaction becomes disfavored at higher temperatures. The solution Haber found was high pressure, forcing the reaction to the side that has fewer molecules of gas, and high temperature to overcome the activation energy barrier and make the reaction kinetically feasible. A complex metal catalysts (magnetite - Fe3O4 -with metal oxides like CaO and Al2O3 which prevent reduction of the Fe with H2) provides an absorptive surface to bring reagents together and facilitate bond breaking in H2 and N2.
We have seen that nature has seemed to involved mechanisms involving the very strange oxygen evolving complex of Mn, Fe, S and Ca to oxidize another very stable and ubiquitous molecule, H2O. Now we explore the amazing mechanisms behind the nitrogenase complex which fixes N2 to form NH3.
What might be needed to drive this reaction biologically? You might surmise the list to include:
-
a source of energy, most likely ATP, to drive this difficult reaction and you’d be right;
-
a source of electrons as the N atoms move from an oxidation state of 0 in elemental N to 3- in NH3; this source turns out to be a protein called flavodoxin or ferridoxin. Of course, these electrons also have interesting sources before they were in the electron carriers of these proteins;
-
some pretty amazing metal centers to accept and donate electrons in a controlled way; these centers are mostly FeS clusters with an additional cluster containing molybdenum (Mo). The clusters are named F, P, and M
-
a source of hydrogen; you might have guessed correctly that it’s not H2 gas (from where would that come?), but H+ ions which are pretty ubiquitously available.
-
a net reaction that is different that the Haber-Bausch process (N2 + 3H2 → 2 NH3).
Here is the actual reaction catalyzed by nitrogenase:
N2 + 8e- + 16ATP + 8H+ → 2NH3 + H2 + 16ADP + 16Pi.
Let’s think a bit about the reaction. As electrons are added the attractions between the nitrogen atoms must decrease. Eventually bonds between them must be broken. Protons could be easily added to maintain charge neutrality. A basic mechanism might involve intermediates as shown below:
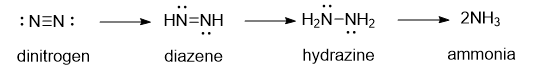
Nitrogenase can also other small molecules with triple bonds, including :C=O: and H-C=C-H.
Navigation
Return to Chapter 8E: Nitrogenase - A Reductive Use of Meta Centers Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8E: Nitrogenase - A Reductive Use of Metal Centers

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.