Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
E: NITROGENASE - A REDUCTIVE USE OF METAL CENTERS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8D:
|
E2. The Structure of Nitrogenase
Nitrogenase is a multiprotein complex containing the following:
-
Two identical subunits, E and F, which have binding sites for the mobile carrier of electrons (the protein ferrodoxin or flavodoxin), ATP, and an FeS cofactor (4Fe-4S, called the F cluster) which accept electrons. These subunits are hence called the nitrogenase reducatase subunits
-
a heterodimer of an alpha and beta subunits. The a (alpha chain) binds the 8Fe-7S F cluster and the Fe-S-Mo M cluster. These subunits comprise the (di)nitrogenase subunits
The overall structure of the protein complex with bound ATP and metal centers are shown below.
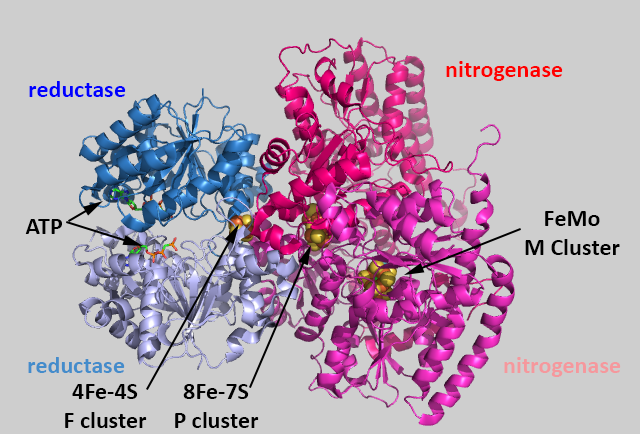
An interactive Jsmol structure of nitrogenase is found in the link below.
![]() JSMol (HTML5)
JSMol (HTML5)
Protopedia: Nitrogenase
An enhanced view of the bound cofactors and ATP are shown in the same spatial orientation in the figure below.
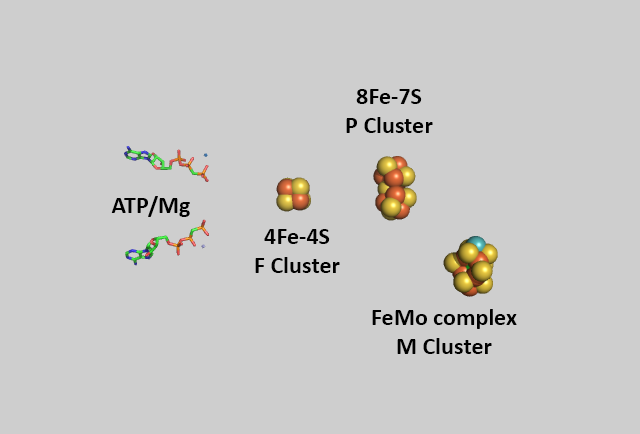
The metal centers are shown below in more detail in both line and space fill views.
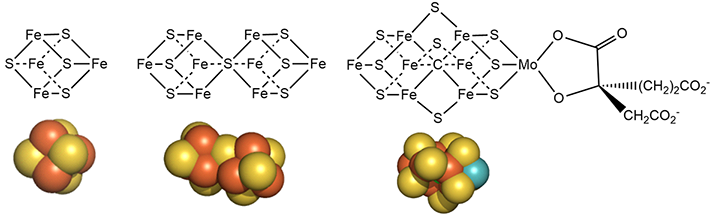
Mo is bound to 3 sulfur ions and two a OH and carboxyl group of 3-hydroxy-3-carboxy, adipic acid in the crystal structure of shown above.
The M cluster has an interstitial carbide ion that derives from -CH3 attached to the sulfur of S-adenosyl-methionine (SAM) allowing the carbide to be labeled with either 13C or 14C for mechanistic studies. These labeled carbides are not exchanged or used as a substrate when the enzyme undergoes catalytic turnover. Hence it seems that the carbide probably just stabilizes the M cluster. it won't be shown in the figures below showing more detailed mechanisms.
Return to Chapter 8E: Nitrogenase - A Reductive Use of Meta Centers Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8E: Nitrogenase - A Reductive Use of Metal Centers

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.