Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 2 - PROTEIN STRUCTURE
H: PROTEIN AGGREGATES AND DISEASE
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 03/10/16
|
Learning Goals/Objectives for Chapter 2H: After class and this reading, students will be able to
|
H3. Misfolding and Aggregation Summary
Recent work has shown the proteins considered to be completely harmless can generate misfolded intermediates that aggregate to produce pre-fibril structures that are toxic to cells. This process is usually prevented in the cell by interaction of nascent forms of the proteins with chaperones, which sequester exposed hydrophobic patches and prevent aggregation. (Obviously prion proteins and the others mentioned above are exceptions). Amyloid fibers (characterized by subunits with an abnormal amount of beta-structure) can be made from many different types of proteins as noted above. Is this property specific to just a handful of proteins, or is it more common than expected from the limited examples noted so far? The new studies show that when a bacterial protein HypF is incubated at pH 5.5 in the presence of trifluoroethanol, aggregates (but not fibrils) form with enhanced beta structure. These aggregates slowly form into fibrils characteristic of amyloid protein fibers. The early aggregates (before fibril formation) proved cytotoxic. Similar results were seen with dimers and trimers (prefibril states) of the amyloid-b peptide released from cultured neurons.
A diverse group of proteins that do not share significant secondary or tertiary structure can form amyloid-like protein aggregates. Even though their monomer forms share little in common, the insoluble amyloid aggregates have a common structure in which the monomer in the aggregates has significant b-structure whose strands run perpendicular to the aggregate axis. Since it has recently been shown that almost any protein, under the "right" set of conditions can form such aggregates, the stabilizing feature of protein aggregates must be potentially found in any protein. Evidence suggests that is the presence of a polypeptide backbone, which can form stable interstrand H-bonds in beta secondary structure, and not the side-chains, that is the source of the common amyloid structure. In contrast, native, nonamyloid forms of normal proteins must arise through specific interactions of unique side chain sequence and structure, which out competes nonspecific interactions among backbone atoms found in amyloid structures. Nonspecific aggregation becomes more prevalent when buried hydrophobic side chains and buried main chain atoms become more solvent exposed. Such exposure occurs when native proteins form intermediate molten globule states when subjected to altered solvent conditions or when destabilizing mutants of the wild-type protein arise. Some mutations may alter the cooperativity of folding which would increases the fraction of nonnative proteins states. Other mutations that decrease the charge on the protein or increasing their hydrophobicity might enhance aggregation. In addition, chemical modifications to proteins (such as oxidation or deamination) might destabilize the native state, leading to the formation of the molten globule state. Once formed, this state may aggregate through sequestering exposed side chain hydrophobes or through inter-main chain H bond formation. Aggregate formation appears to proceed through the initial formation of soluble units (which may or not be more toxic to cells than the final aggregate). Aggregates are kinetically stable species. Since amyloid aggregates are cytotoxic and almost any protein can form them, albeit with different propensities, nature, through evolutionary selection, has presumably disfavored proteins with high tendencies to form such aggregates.
-
 Nature
Insights:
Protein Folding and Misfolding (2003) - Great series of articles
(PDFs).
Nature
Insights:
Protein Folding and Misfolding (2003) - Great series of articles
(PDFs).
Wasmer et al have determined a solid state NMR structure of an amyloid fiber state for a prion forming domain of the HET-s protein from the fungus P. anserina. It consists of a left-handed b solenoid.
![]() Jmol:
Jmol:
![]() Amyloid
Fibril Models from UCLA
Amyloid
Fibril Models from UCLA
Clearly accurate protein folding is required for cell viability. Aberrant protein folding clearly can be the cause of serious illness. Given the extraordinary nature of the task and its failure, the process governing protein folding must be highly regulated. The diagram below shows the steps that determine intracellular concentrations and locations of normal and aberrant protein structures.
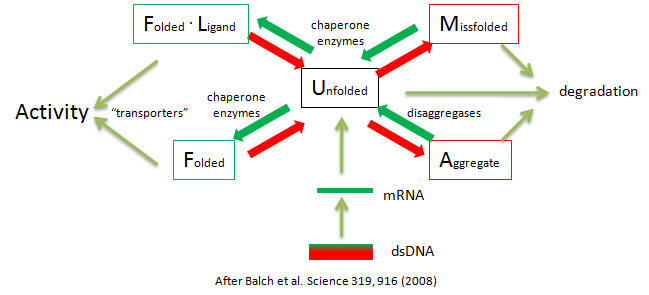
Potential therapies of diseases of proteostasis include replacing aberrant proteins, shifting the equilibria toward active forms with small ligands, or modulating the pathways with agents that influence pathways such as signal transduction, transcription, translation, degradation, and translocation using molecules like siRNAs to modulate concentrations of chaperons, disaggregases, and signal pathways.
Navigation
Return to Chapter 2H: Protein Aggregates and Disease
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 2H: Protein Aggregates and Disease

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.