Biochemistry Online: An Approach Based on Chemical Logic

MOLECULAR MECHANICS AND DYNAMICS
04/13/16
D. Bonded Interaction Energy
The mathematical form of the energy terms varies from force-field to force-field. The more common forms will be described.
Stretching Energy: Estretch = Σbondskb (r - ro)2
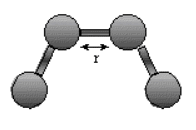
The stretching energy equation is based on Hook's law. The kb parameter defines the stiffness of the bond spring. R0 is the equilibrium distance between the two atoms. It should make sense that deviations from the equilibrium length would be associated with higher energy. The E vs r curves is hence a parabola:
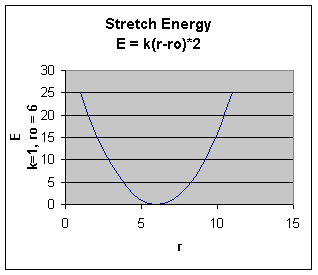
Obviously only small changes in r are allowed as to large an r value would lead to bond breaking.
Bending Energy: Ebending = Σangles kΘ (Θ - Θo)2
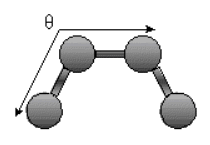 .
.The bending energy equation is also based on Hook's law. The kΘ parameter controls the stiffness of the angle spring, while the Θ0 is the equilibrium angle. As above, the graph of E vs theta is expected to be a parabola.
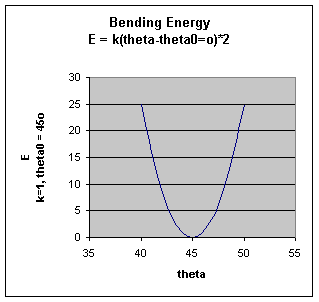
.
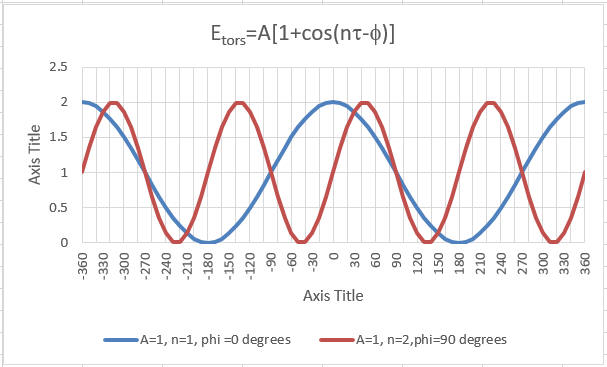
Torsion Energy Etorsion = Σtorsions A [1 + cos ( ntau - Θ) ]
The torsion energy is modeled by a periodic function, much as you have seen with energy plots associated with Newman projections sighting down C-C bonds fro butane, for example.
![]() Wolfram
Mathematica CDF Player - Torsional Energy (free
plugin required)
Wolfram
Mathematica CDF Player - Torsional Energy (free
plugin required)
The parameters (determined for different 4 bonded atoms small molecules using curve fitting) for these are:
- amplitude A
- periodicity n (ethane, sighting along the C-C axis in a Newman projects displays a periodicity of 120 degrees)
- phase shift Phi: shifts curve along rotation (tau) axis. parameter controls its periodicity, and phi shifts the entire curve along the rotation angle axis (tau).
![]() Interactive SageMath
Graph: Interactive Graph of 6-12 Lennard Jones Potential
Interactive SageMath
Graph: Interactive Graph of 6-12 Lennard Jones Potential
Navigation
Return to Molecular Mechanics and Dynamics Contents
Return to Biochemistry Online Table of Contents
Archived version: Molecular Mechanics and Dynamics

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.