Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - METABOLIC AND SIGNAL TRANSDUCTION
A. ACTIVE TRANSPORT
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9A:
|
A4. Transport of Protons
Driven by oxidation - The proton gradient formed during aerobic oxidation and photosynthesis in mitochondria and chloroplast, respectively, is paid for by free energy decreases associated with oxidation of organic molecules.
Driven by ATP cleavage - As mentioned above, protons are transported into the the lumen of the stomach by a K+-H+ ATPase.
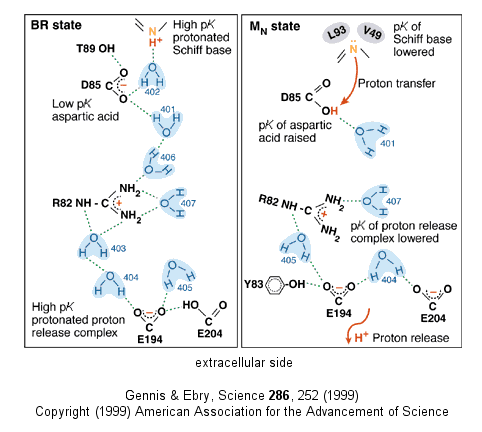
Driven by light - Photosynthetic bacteria have a membrane protein called bacteriorhodopsin which contains retinal, a conjugated polyene derived from beta-carotene. The retinal is covalently attached to the protein through a Schiff base linkage to an epsilon amino group of Lys (much as pyridoxal phosphate is in PLP-dependent enzymes). Bacteriorhodopsin is analogous to the visual pigment protein rhodopsin in retinal cells. Absorption of light by the retinal induces a conformational changes in the all trans-retinal, which causes an associated conformational change in bacteriorhodopsin. The initial state (BR) changes through a series of intermediates (K, L, M, N, and O). Various side chains and the protonated N of the Schiff base of retinal change their relative positions with respect to each other, which leads to changes in protonation states of the side chains and ultimately vectorial discharge of protons through the membrane. As the M state forms, H+ is moved to the extracellular side of the membrane (as shown below). Later a H+ is taken up on the cytoplasmic side (at the Schiff base of the retinal link) leading to reformation of the BR state. Experiments have been done to trap the protein in some of these intermediate states. In one (Leuke et al, 1999), a mutant (Asp 96 to Asparagine or D96N) trappped the protein in a state, MN, that occurs after a H+ has been moved to the extracellular side but before a compensatory H+ has been taken up on the cytoplasmic face. The mutation hinders the reuptake of the proton.
![]() Animation
of bacteriorhodopsin
Animation
of bacteriorhodopsin
Figure: BACTERIORHODOPSIN AND PROTON TRANSPORT
Figure: A NEW VERSION SHOWING
PROTON TRANSFER IN BACTERIORHODOPSIN
![]() Jmol:
Updated Bacteriorhodopsin Crystallized From Bicelles
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Updated Bacteriorhodopsin Crystallized From Bicelles
Jmol14 (Java) |
JSMol (HTML5)
Navigation
Return to Chapter 9A: Active Transport Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9A: Active Transport

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.