Bacteriorhodopsin(bR) Crystallized From Bicelles
Java version
HTML 5 version (does not require Java; downloads and moves
slowly)
Introduction
"bR is a light driven proton pump from Halobacterium salinarum and contains a covalently bound retinal chromophore that gives it its purple color.
bR occurs naturally as a two-dimensional crystal in the inner membrane, forming a specialized membrane structure called the purple membrane.
In the purple membrane, the protein subunits are organized in trimeric units which is the form seen in the layers of the crystals grown from the lipid cubic phase."(Faham)
For more information see
Biochemistry Online: Chapter
9A - Energy Transduction: Uses of ATP
Wire Frame
Protein Backbone
The backbone here is colored according to its anisotropic temperatures, which is a measure of an atom's mobility. Red indicates greater mobility, and blue indicates less mobility. The absence of red indicates the relative immobility of this protein in the lipid layer.
Cartoon with hydrogen bonds
In this rendering, the protein can be seen as a monomer with 7 helices and an antiparallel beta sheet.
Retinal(spacefill) Bound to Protein(wireframe)
Retinal(spacefill) Bound to Protein(cartoon)
With the Retinal in spacefill mode, it becomes easier to see how close it is to the protein.
Retinal Alone
"Retinal is a conjugated polyene derived from beta-carotene. It is analogous to the visual pigment protein rhodopsin in retinal cells. Absorption of light by the retinal induces a conformation changes in the retinal and protein, which leads to vectorial discharge of protons."(Jakubowski)
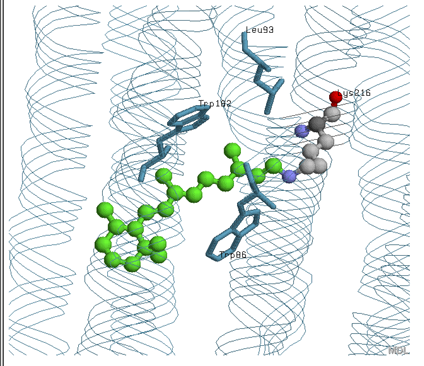
Retinal active site
According to the picture above and the retinal active site depicted in the chime, the retinal is contacted by Leu 93, Trp 182, and Trp 86 through different interactions which are probably hydrophobic. It is also supposed to be covalently linked to Lys 216.
Water binding Regions
Notice that the water molecules are located primarily at the turns of the helices. This is important because those parts are more likely to be outside of the hyrdrophobic area of the bilayer. Also, the placement of the water matches well with the lipophilicity surface potential below. However, there are some water molecules located in between the helices. The importance of this is discussed below.
Proton Pumping
Retinal Proton Pumping
The amino acids in this rendering are important to the proton pumping model. The water is also an important factor in the bacteriorhodopsin's proton pumping because it provides stability through H-bonding and protons.