Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
C: SIGNALING PROTEINS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9C:
|
Estonian Translation √ by Anna Galovich
C11. Phosphatases
There are three main families of phosphatases, the phospho-Tyr phosphatases (PTP), the phospho-Ser/Thr phosphatases, and those that cleave both. Of all phosphorylation sites, most (86%) are on Ser, 12% involve Thr, and about 2% on Tyr. They can also be categorized by the molecular sizes, inhibitors, divalent cation requirements, etc. In contrast to kinases which differ in the structure of their catalytic domains, many phosphatases (PPs below) gain specificity by binding protein cofactors which facilitate translocation and binding to specific phosphoproteins. The active phosphatase hence often consists of a complex of the phosphatase catalytic subunit and a regulatory subunit. Regulatory subunits for Tyr phosphatases may contain a SH2 domain allowing binding of the binary complex to autophosphorylated membrane receptor Tyr kinases.
Important Ser/Thr phosphatases (PPs for Protein Phosphatases) include:
-
Protein phosphatase 1 (PP-1 or Ppp1) - This is the most abundant PPPs in humans. Different regulatory subunits target this to the liver glycogen particles (GL subunit), striated muscle glycogen and sacroplasmic reticulum (GM subunit) or smooth muscle fibers (M subunit). It is also present in the nucleus where it is presumably involved in regulation of transcription factors. It is also involved in RNA splicing and signaling at neural synapses.
-
Protein phosphatase 2A (PP-2A or Ppp2) - is a trimer with catalytic, regulatory, and a scaffolding (also regulatory) structural subunits. It is found mainly in the cytoplasm and is involved in a myriad of cellular process.
-
Protein phosphatase 2B (PP-2B or Ppp3) - also called calcineurin or Ca2+/Calmodulin dependent protein phosphatase - It consists of a catalytic subunit (calcineurin A) and a regulatory, calcium-binding subunit (calcinerin B). It is inhibited by the complex of the immunosuppressant cyclosporin and FK506 with immunophilins. PP2B regulates PKA and PKC
PP1, 2A and 2B share a great deal of amino acid homology, and based on this homology, belong to one family. PP2C belongs to another. PPs are often categories into three families including, phosphoprotein phosphatases (PPPs) and metal-dependent protein phosphatases (PPMs). There about 30 catalytic PP subunits (many fold fewer than Ser/Thr Kinases). They gain specificity by binding numerous modulatory regulatory subunits.
As with other proteins, the names given to the proteins when discovered often do not reflect an organization scheme that would name different members based on structural similarities. PP-1, 2A, and 2B are better named Ppp1, Ppp2, and Ppp3 which denotes member of the Protein PP (PPP) family. PP-2C would be named Ppm1 as the first member of the PPN family. All PPPs have three short sequence motifs that bind divalent cations.
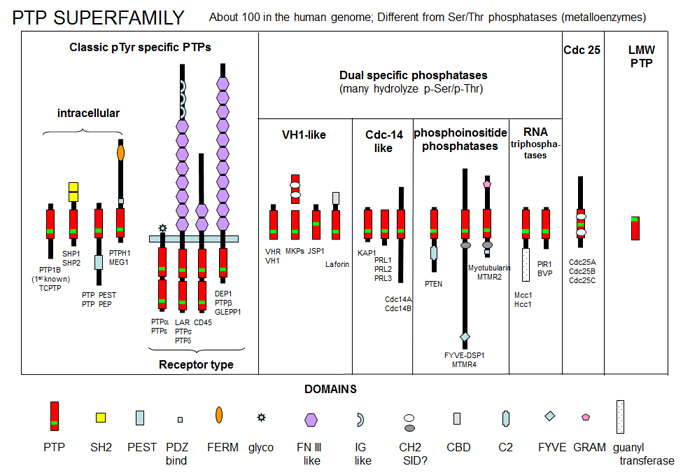
Protein Tyr phosphatases (PTPs) consist of receptor-like (transmembrane) and intracellular Tyr phosphatases. They more resemble tyrosine kinases in their complexity than the Ser/Thr phosphatases. There are about 100 PTPs in the genome, a number similar to the number of protein tyrosine kinases. PTPs have an active site Cys in a CX5R-(S/T) motif with an active site Cys nucleophile and an Arg in the phosphate binding (P) loop. Important examples include:
-
PTP1B - dephosphorylates many cell surface receptors (insulin, EGF, PDGF) that have been phosphorylated on Tyr residues. Its main activity seems to dephosphorylate nascent receptors in the endoplasmic reticulum before they get to the final cell membrane destination.
-
Low molecular weight PTPase - These have roles in metabolism and differentiation of cells. They have a molecular weight of 18,000 and have an active site CX5R-(S/T) motif, where the C (Cys) is an active site nucleophile.
Figure: PTP Super Family
Web Links for Phosphatases
![]() Nontransmembrane
and Receptor-Like Protein Tyrosine Phosphatases
Nontransmembrane
and Receptor-Like Protein Tyrosine Phosphatases
Navigation
Return to Chapter 9C. Signaling Proteins Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9C: Signaling Proteins

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.