Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
C: SIGNALING PROTEINS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9C:
|
Estonian Translation √ by Anna Galovich
C4. Receptor Tyrosine Kinases (RTK)
Cascade of events: A transmembrane receptor WITH HORMONE-DEPENDENT ENZYMATIC ACTIVITY (tyrosine kinase) binds an extracellular chemical signal, causing a conformational change in the receptor which propagates through the membrane. The intracellular domain of the receptor becomes an active tyrosine kinase which can phosphorylate itself (autophosphorylation) or other proteins. Such kinases are usually active in a multimeric state. Typically, binding of two molecules of a ligand or a ligand dimer to individual subunits of the receptor causes the monomers of the receptor to dimerize. In this form the kinase activity of the receptor is activated. The individual subunits of the multimer are proteins with a single transmembrane helix. Examples are the insulin receptor and epidermal growth factor receptor.
Receptor Tyr kinases autophosphorylate themselves, in a process required for their activity. When the receptor is autophosphorylated, other proteins can bind to the cytoplasmic domain of the receptor Tyr kinase where they are phosphorylated. The target substrates phosphorylated by the receptor Tyr kinase are proteins with a common 100 amino acid domain called SH for src homolgy, based on structural homology to another cytoplasmic protein, Src. Src is an intracellular Tyr kinase activated when it binds through 2 SH domains to the autophosphorylated receptor Tyr kinase. Specifically, the SH2 domain has been shown to bind phosphorylated peptides. These domains target proteins to the autophosphorylated receptor Tyr kinase.
Figure: Receptor/Ligand-Dependent Protein Kinases
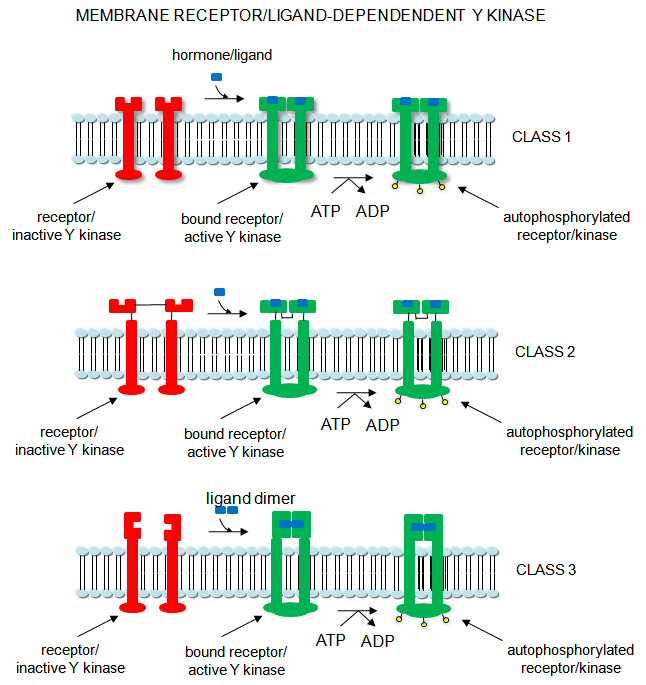
Many proteins involved in signal transduction have SH2 domains. Some of these proteins also have catalytic domains with kinase activity. Others have phosphatase, transcription factor. or scaffolding domains.
.
Navigation
Return to Chapter 9C. Signaling Proteins Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9C: Signaling Proteins

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.