Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
C: SIGNALING PROTEINS
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9C:
|
Estonian Translation √ by Anna Galovich
C7. Small G proteins, GAPs and GEFs
In the previous sections , the small GDP/GTP binding protein Ras was introduced. Mammalian cells contain 3 variants of Ras: H, K, and N. They all bind GDP/GTP and have GTPase activity. Ras is targeted to the cell membrane through the post-translational addition of a hydrophobic farnesyl group. When activated by binding to GTP, it can bind to and activate a protein call Raf-1, which is on binding become an active tyrosine kinase. Ras has intrinsic GTPase activity, so eventually active Ras will deactivate itself.
Ras is just one member of a large superfamily of small G protein, which all have GTPase activity. However, they are poor GTPases, so they nees help to autocatalytically cleave the GTP to GDP.They are so critical for cell function that two other classes of proteins, GAPs and GEFs exist to regulate their activity by modulating the balance of bound GTP (active form of the protein) and GDP (inactive form of the small G protein).
- GAP - GTPase Activating Proteins: These proteins facilitate hydrolysis of bound GTP hence down regulating the activity of the G protein.
- GEF - GTP Exchange Factors: These proteins facilitate GDP to GTP exchanges, hence activating the G protein.
Before describing these proteins, we need to have a better understanding of the family of small G proteins.
Small G proteins
The superfamily of small G proteins have a common 20K molecular weight catalytic (GTPase) domain with 5 alpha helices, 6 beta strands and connecting loops. The small G proteins are "active" in the GTP bound form. Hydrolysis of GTP to GDP causes the protein to become inactive.
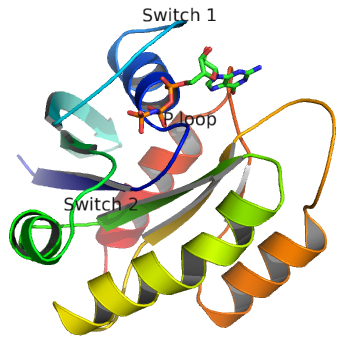
Important parts of Ras necessary for GTP binding:
- phosphate-binding (P loop), residues 10 to 16 (dark blue trace below);
- switch regions I (30 to 37, light blue trace) and II (60 to 76, green trace), which are flexible loops which sandwiches GTP.
These are shown in the image and Jsmol below.
![]() Jmol:
H-Ras:GTP complex (5p21)
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
H-Ras:GTP complex (5p21)
Jmol14 (Java) |
JSMol (HTML5)
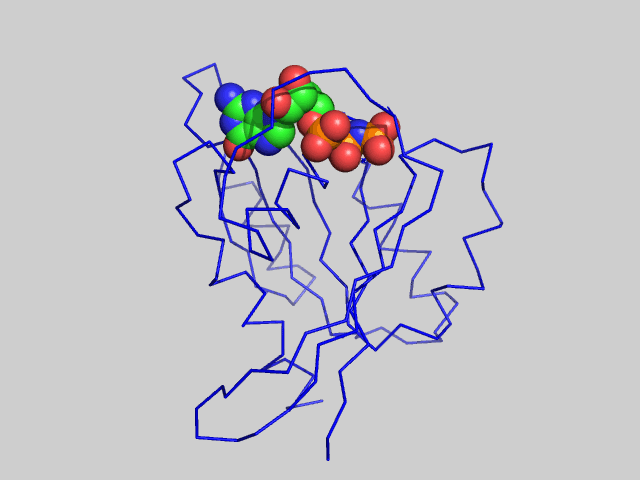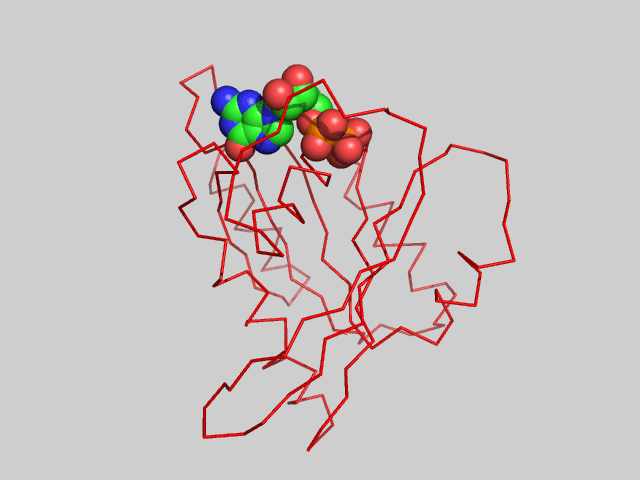
The animated figure below shows structural differences between the GTP bound form (blue, pdb id 5p21) and GDP form (red, pdb id 4q21) of the H-Ras protein. One helix and nearby loops are perturbed.
There are many distinct types of small G proteins, comprising a family of about 150 in humans. Here are some with select target proteins they bind to and regulate in a multiple of signal transduction pathways. A brief description of target binding proteins, taken from UniProt, is also given.
Arf (Q8N726 (ARF_HUMAN): Capable of inducing cell cycle arrest in G1 and G2 phases. Acts as a tumor suppressor. Binds to MDM2 and blocks its nucleocytoplasmic shuttling by sequestering it in the nucleolus. This inhibits the oncogenic action of MDM2 by blocking MDM2-induced degradation of p53 and enhancing p53-dependent transactivation and apoptosis. Also induces G2 arrest and apoptosis in a p53-independent manner by preventing the activation of cyclin B1/CDC2 complexes. Binds to BCL6 and down-regulates BCL6-induced transcriptional repression. Binds to E2F1 and MYC and blocks their transcriptional activator activity but has no effect on MYC transcriptional repression. Binds to TOP1/TOPOI and stimulates its activity. This complex binds to rRNA gene promoters and may play a role in rRNA transcription and/or maturation. Interacts with NPM1/B23 and promotes its polyubiquitination and degradation, thus inhibiting rRNA processing. Interacts with COMMD1 and promotes its 'Lys63'-linked polyubiquitination. Interacts with UBE2I/UBC9 and enhances sumoylation of a number of its binding partners including MDM2 and E2F1. Binds to HUWE1 and represses its ubiquitin ligase activity. May play a role in controlling cell proliferation and apoptosis during mammary gland development. Isoform smARF may be involved in regulation of autophagy and caspase-independent cell death; the short-lived mitochondrial isoform is stabilized by C1QBP : used in vesicular proteins trafficking;
Ran (P62826 (RAN_HUMAN): GTPase involved in nucleocytoplasmic transport, participating both to the import and the export from the nucleus of proteins and RNAs. Switches between a cytoplasmic GDP- and a nuclear GTP-bound state by nucleotide exchange and GTP hydrolysis. Nuclear import receptors such as importin beta bind their substrates only in the absence of GTP-bound RAN and release them upon direct interaction with GTP-bound RAN while export receptors behave in the opposite way. Thereby, RAN controls cargo loading and release by transport receptors in the proper compartment and ensure the directionality of the transport involved in protein import/export into the nucleus as well as RNA export;
Rab (Ras analog in brain, P62491 (RB11A_HUMAN) ): GDP-bound form and an active GTP-bound form that is able to recruit to membranes different set of downstream effectors directly responsible for vesicle formation, movement, tethering and fusion. That Rab regulates endocytic recycling. Acts as a major regulator of membrane delivery during cytokinesis. Together with MYO5B and RAB8A participates in epithelial cell polarization. Together with RAB3IP, RAB8A, the exocyst complex, PARD3, PRKCI, ANXA2, CDC42 and DNMBP promotes transcytosis of PODXL to the apical membrane initiation sites (AMIS), apical surface formation and lumenogenesis. Together with MYO5B participates in CFTR trafficking to the plasma membrane and TF (Transferrin) recycling in nonpolarized cells. Required in a complex with MYO5B and RAB11FIP2 for the transport of NPC1L1 to the plasma membrane. Participates in the sorting and basolateral transport of CDH1 from the Golgi apparatus to the plasma membrane. Regulates the recycling of FCGRT (receptor of Fc region of monomeric Ig G) to basolateral membranes. May also play a role in melanosome transport and release from melanocytes regulate vesicular trafficking pathways. ;
Rho (P61586 (RHOA_HUMAN): Regulates a signal transduction pathway linking plasma membrane receptors to the assembly of focal adhesions and actin stress fibers. Involved in a microtubule-dependent signal that is required for the myosin contractile ring formation during cell cycle cytokinesis. Plays an essential role in cleavage furrow formation. Required for the apical junction formation of keratinocyte cell-cell adhesion. Stimulates PKN2 kinase activity. May be an activator of PLCE1. Activated by ARHGEF2, which promotes the exchange of GDP for GTP. Essential for the SPATA13-mediated regulation of cell migration and adhesion assembly and disassembly. The MEMO1-RHOA-DIAPH1 signaling pathway plays an important role in ERBB2-dependent stabilization of microtubules at the cell cortex. It controls the localization of APC and CLASP2 to the cell membrane, via the regulation of GSK3B activity. In turn, membrane-bound APC allows the localization of the MACF1 to the cell membrane, which is required for microtubule capture and stabilization. Regulates a signal transduction pathway linking plasma membrane receptors to the assembly of focal adhesions and actin stress fibers. Involved in a microtubule-dependent signal that is required for the myosin contractile ring formation during cell cycle cytokinesis. Plays an essential role in cleavage furrow formation. Required for the apical junction formation of keratinocyte cell-cell adhesion. May be an activator of PLCE1. Activated by ARHGEF2, which promotes the exchange of GDP for GTP. Essential for the SPATA13-mediated regulation of cell migration and adhesion assembly and disassembly. The MEMO1-RHOA-DIAPH1 signaling pathway plays an important role in ERBB2-dependent stabilization of microtubules at the cell cortex. It controls the localization of APC and CLASP2 to the cell membrane, via the regulation of GSK3B activity. In turn, membrane-bound APC allows the localization of the MACF1 to the cell membrane, which is required for microtubule capture and stabilization (By similarity). Regulates KCNA2 potassium channel activity by reducing its location at the cell surface in response to CHRM1 activation; promotes KCNA2 endocytosis involved in cytoskeleton reorganization. a mitochondrial version is involved in mitochondrial trafficking ;
Ras: interacts with PLCE1 (PubMed:11022048). Interacts with TBC1D10C (PubMed:17230191). Interacts with RGL3 (By similarity). Interacts with HSPD1 (By similarity). Found in a complex with at least BRAF, HRAS, MAP2K1, MAPK3 and RGS14 (By similarity). Interacts (active GTP-bound form) with RGS14 (via RBD 1 domain) (By similarity). Forms a signaling complex with RASGRP1 and DGKZ (PubMed:11257115). Interacts with RASSF5 (PubMed:18596699). Interacts with PDE6D (PubMed:11980706). Interacts with IKZF3 (PubMed:10369681). Interacts with RACK1 (PubMed:14500341). Interacts with PIK3CG; the interaction is required for membrane recruitment and beta-gamma G protein dimer-dependent activation of the PI3K gamma complex PIK3CG:PIK3R6 (By similarity). Interacts with RAPGEF2 found in many signal transduction pathways; Plays an important role in the regulation of cell proliferation (PubMed:23698361, PubMed:22711838). Plays a role in promoting oncogenic events by inducing transcriptional silencing of tumor suppressor genes
Small G proteins are a fundamental form of molecular switch. To add to the complexity, there are about 150 in the human genome. They are too important to not be regulated. Probably the most common mutation in human cancer cells involved a single amino acid changes in Ras (H, K and N form). If the GTPase activity is inhibited with mutation, the protein may be constitutively active. Such a Ras mutation is found in almost 90% of pancreatic cancers. Hence researchers have been trying to design drugs that inhibit its GTPase activity. This has proven difficult since it has very few targetable pockets that could bind a drug.
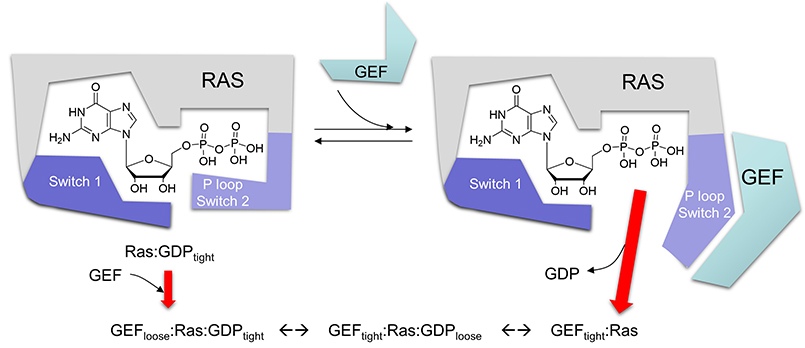
Regulation of small G proteins: GAPs and GEFs
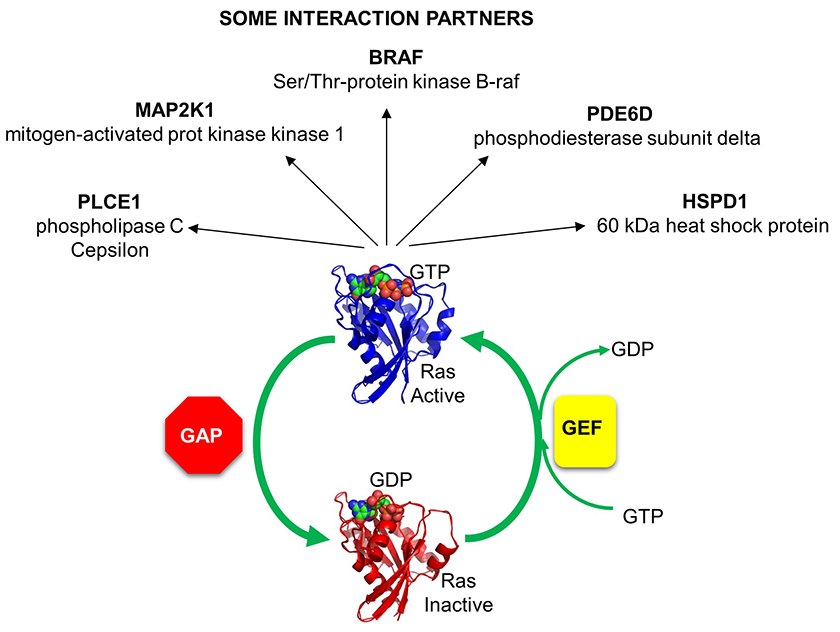
Given the critical importance of small G proteins, it makes biological sense that their on/off activity would be exquisitely regulated. Indeed, they are. Two families of protein have evolved to regulate them by determining whether GTP or GDP is bound to the protein (leading to an active, and inactive small G protein respectively). One family, GTPase activating proteins (GAPs) facilitate the hydrolysis of bound GTP, leading to the inhibition of the protein. The other proteins are GTP exchange proteins (GEFs), which facilitate the exchange of GTP for GDP, activating the protein.
The activity of Ras GAPs and GEFs, as well as various proteins interacting with Ras, are depicted in the figure below.
GAPs - GTPase Activating Proteins
The hydrolysis of the gamma phosphate of GTP by water in Ras proceeds by a pentavalent transition state with two axial and three equatorial ligands to the P. Developing charge in the transition state would usually be stabilized by catalytic residues in the catalytic domain of Ras. However, Ras is a poor GTPase. There's were GAP comes in. In the Ras/GAP complex, GAP positions its Arg 789 on the GAP in a position to stabilize the transition state for Ras-bound GTP cleavage. This Arg 789 is almost in the same position as Arg 178 in the Galpha inhibitor subunit of a heterotrimeric G protein which inhibits GPCR signaling. Both of these arginines have similar catalytic function.
![]() Jmol:
H-Ras:Ras GAP complex (1wq1)
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
H-Ras:Ras GAP complex (1wq1)
Jmol14 (Java) |
JSMol (HTML5)
GEFs - GTP Exchange Factor
Once bound to Ras, GDP dissociates very slowly. Values of 10-5 sec-1 have been reported for first order rate constant of the dissociation of GDP from a small G protein. Assuming that the diffusion controlled on rate constant for the complex, the KD for the G protein:GDP complex would be 0.1 pM and the half life would be 0.8 days, similar to the lac repressor:DNA operator complex. Hence the protein, when bound to GTP, is essentially locked in the off position. What if it needs to be reactivated in a quick fashion? How can the rate at which GDP dissociates be increased so that GTP could replace it? If it were to dissociate, GTP could quickly replace it since from an equilibrium point of view, Ras and other small G proteins would favor GTP binding since its concentration in the cell is higher.
One could envision a number of ways to change the rate at which GDP dissociates. In organic chemistry, a favorite students answer to many questions is to envoke steric effects. In biochemistry, the analog is often conformational changes. How could you change the conformation of Ras such that it might favor GTP binding? That could occur by ligand binding or more likely by a post-translation modification such as phosphorylation as part of a signaling process. It turns out that for the case of small G proteins, another mechanism is evoked: the binding of another protein, a GTP Exchange Factor or GF, which promotes GTP exchange for the bound GDP. If the Ras:GEF:GDP complex has a 10,000 increase in koff for GTP, the half life of the bound GDP is 7 seconds.
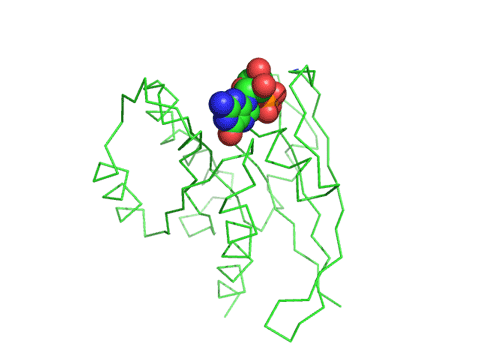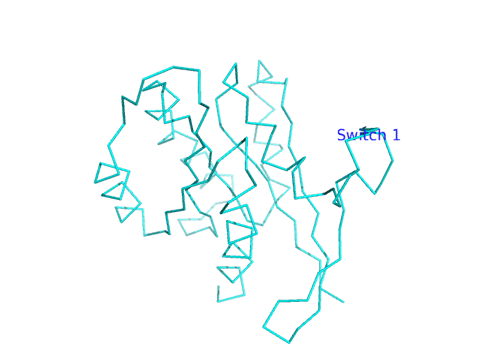
The crystal structure of a Ras GEF, SOS, in complex with Ras allows a detailed understanding of the mechanism. SOS, a cytoplasmic protein, is recruited to the cell membrane where active Ras is found, teathered to the membrane with a fanesyl hydrophobic attachment.
![]() Jmol:
H-Ras:SOS (a GEF) complex (1bkd)
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
H-Ras:SOS (a GEF) complex (1bkd)
Jmol14 (Java) |
JSMol (HTML5)
A better view of the conformational change induced on binding of SOS to Ras is shown in the images and Jmol files below which show the difference in Ras structures between the Ras:GDP complex (pdb id 4q21) and the Ras alone in the Ras:SOS (pdb id 1bkd) complex). Note the large shift in Switch 1 in the Ras structure from the Ras:SOS complex. This leaves a "gaping" hole from which GDP can "escape".
Navigation
Return to Chapter 9C. Signaling Proteins Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9C: Signaling Proteins

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.