Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
D: mTOR and Nutrient Signaling
BIOCHEMISTRY - DR. JAKUBOWSKI
Last Update: 6/22/16
|
Learning Goals/Objectives for Chapter 9D:
|
D4. Regulation of mTORC1 by Leucine
mTORC1 clearly is a key regulator of protein synthesis but that begs the question as to how it determines that protein synthesis is required. How does it sense that? Obviously regulators of mTORC1 might be amino acids in cells, but who would have thought that the master regulator would be leucine, a simple branched chain hydrophobic amino acid.
It would be nice if free leucine bound directly to mTORC1, but it's not that simple. Rather, it binds to a "leucine" receptor, sestrin 2 (SESN2). The figure below shows the binding interactions between Leu (spacefill) and key side chains in sestrin 2 (5dj4).
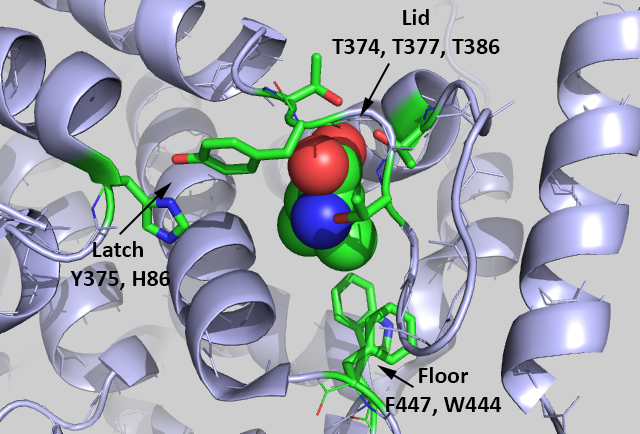
The Leu is rather buried, which suggests a conformational change ensues on binding to the protein. Saxton et al (2016) describe three types of sestrin2 side chains involved in the interaction:
Lid: Thr374, Thr377, and Thr386 form H bonds with the Leu amine and carboxyl group. Leucine is represented as a stick model (orange).
Latch: Tyr375 and His86 forms hydrogen bonds to the Leu. Note that these residues are distal in the chain and are probably pulled together during the conformational changes which occurs after binding to form a latch to sequester the bound Leu.
Floor: F447 and W444 which interact with the nonpolar side chain of Leu.
![]() Jmol:
Sestrin 2
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Sestrin 2
Jmol14 (Java) |
JSMol (HTML5)
What happens after leucine binds? It's a complicated but understandable process described below in words and an images. But first a quick review. Kinases must be regulated to be turned on and off at the right time. They are often regulated by phosphorylation, as mTOR is. In addition, they can be regulated by binding proteins as mTOR is (by Raptor, FKBP, etc). They can also be regulated by small G proteins (like Ras) which are active when bound to GTP and inactive when bound to GDP. Of course whether small G proteins have GTP bound depends in part if they interact with GAPs (GTPase activating protein which inactivates small G proteins) or GEFs (which facilitate exchange of GDP for GTP and activate them). ISuch a master regulator of growth as mTORC1 is regulated by all of these, in addition to the presence of abundant leucine.
In the absence of leucine, sestrin 2 is bound to a protein called GATOR2 (GTPase-activating protein - GAP - activity toward Rags 2). The binding of leucine to sestrin 2 causes the dissociation of GATOR2. This is shown in the figure below;
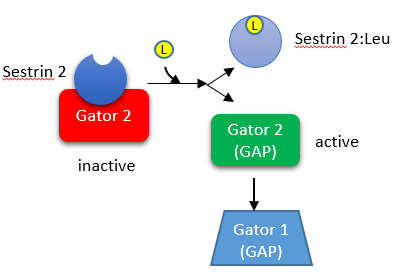
Free GATOR2 is a GAP that regulated mTORC1. Specifically, it regulates the activity of a heterodimer of small GTP binding proteins, RagA/B:RagC/D (see pathway above) which are associated with the outer leaflet of the lysosome. There they interact with a membrane protein, SLC38A9 and a protein that regulates the Rag proteins, which of course is named Ragulator. Active RagA/B:RagC/D recruits mTORC1, presumably through the Raptor subunit) from the cytoplasm to the lysosome membrane. Small G proteins like Ras, when activated by exchanging bound GDP for GTP, can interact with and activate kinases (like the Raf kinase for Ras). When mTORC1 binds to active RagA/B:RagC/D, it becomes activated.
We often think of activating a protein by ligand binding, which promotes a conformational change, or by post-translational modification, which can provide a binding interaction or conformational change to activate the protein. Another way is to inhibit an inhibitor of a protein, as shown below. Y inhibits Z as denoted by the blunt ended arrow. If X inhibits Y, the Y can't inhibit Z, which is now active. This is analogous to the quote that "the enemy of my enemy is my friend", which has been attributed to Kautilya (from India) in the 4th century BCE.

Leucine binding to sestrin 2 leads to free GATOR, which activates mTORC1 by blocking downstream inhibitors as shown in two different figures below.
The figure immediately below (after Buel and Blenis, 2016) shows the interactions from an activation (arrow) or inhibition (blunt arrow) perspective.
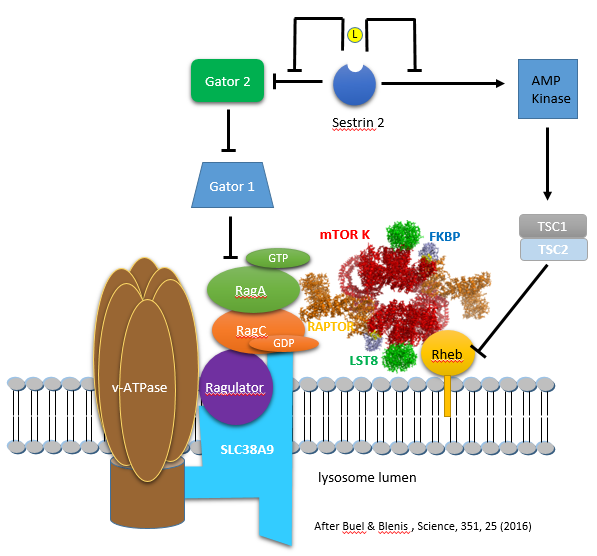
The figure above shows the involvement of multiple proteins in the lysosome membrane that are involved in mTORC1 activation. There is yet another way that the RagA/B and RagC/D protein are regulated (other than by the GATOR GAP activity. The main one appears to be Ragulator, which is a GEF for the Rag proteins. Here is a summary of components of this lysosomal membrane recruitment center for mTORC1.
- Ragulator (what a great name) binds and recruits the small G proteins Rag to the lysosome membrane where Ragulator acts as a GEF for RagA/B
- SLC38A9 is a weak amino acid transporter in the lysosome membrane, with preferences towards polar amino acids. More likely it is yet another sensor of amino acids, particularly of arginine, which has a high concentration in the lysosome. The protein has a high Km for transport of Arg. It has a Ragulator binding domain and is hence part of the complex that recruits mTORC1 to the lysosome
- vacuolar adenosine triphosphatase (v-ATPase): function unclear
These interactions, which involve multiple activations and inhibitions, are difficult to follow even with a diagram. The actions of small G proteins can be especially difficult to understand since the G protein is biologically INACTIVE in its GDP-bound form towards its target binding protein. This occurs when the GTPase activity of the G protein is ACTIVE. The arrows and blunt end arrows in the figure above represent the activity of the protein towards its target protein.
Here are two alternative ways to make sense out of the interactions:
- Stepping backwards from Rag A/B, Gator 1 (a GAP) inhibits the ACTIVITY of the protein Rag A/B as it acts as a GAP to leave Rag A/B in the inactive GDP-bound state. Paradoxically this occurs as the inherent GTPase activity of the protein is activated as described above). Free Gator 2 (also a GAP) appears to inhibit the GAP activity of Gator 1 (through an unknown mechanism), thereby increasing the amount of GTP-bound Rag A/B, which then can activate mTORC1. Free Gator 2 does this only if Sestrin 2 is bound to Leu which allowed the Gator 2 to dissociate from the inactive sestrin 2:Gator 2 complex.
- The diagram above shows that in the absence of leucine, three blunt end (inhibition) arrows occur between Sestrin 2 and Rag A/B. One blunt arrow denotes inhibition, two activation (inhibition of inhibition), and hence three net inhibition Hence in the absence of Leu (when Sestrin is bound to Gator 2, Rag A/B is inhibited in its ability to activate mTORC1 as Rag A/B is in the GDP-bound state. However, free leucine unblocks the inhibitor action of sestrin 2 as Gator 2 is now free and active on its own.
Amino acids (especially arginine which is abundant) in the lumen of the lysosome activate, through the v-ATPase and SLC38A9, the GEF activity of Ragulator. When Rag A/B has sufficient GTP, some conformational changes must ensue to allow mTORC1 recruitment to the lysosomal membrane.
Navigation
Return to Chapter 9D. Nutrient Signaling Sections
Return to Biochemistry Online Table of Contents

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.