Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 6 - TRANSPORT AND KINETICS
C: ENZYME INHIBITION
BIOCHEMISTRY - DR. JAKUBOWSKI
06/12/2014
|
Learning Goals/Objectives for Chapter 6C: After class and this reading, students will be able to
|
C7. Inhibition by Temperature and pH Changes
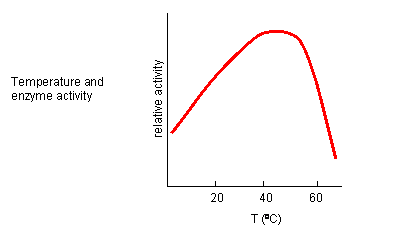
From 0 to about 40-50o C, enzyme activity usually increases, as do the rates of most reactions in the absence of catalysts. (Remember the general rule of thumb that reaction velocities double for each increase of 10oC.). At higher temperatures, the activity decreases dramatically as the enzyme denatures.
Figure: Temperature and Enzyme Activity
INHIBITION BY PH CHANGE
pH has a marked effect on the velocity of enzyme-catalyzed reactions.
Figure: pH and Enzyme Activity
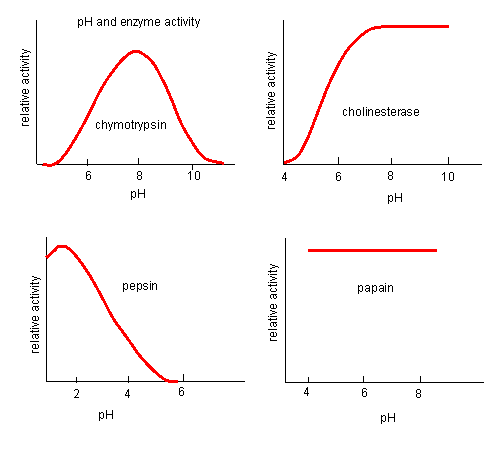
Think of all the things that pH changes might affect. It might
- affect E in ways to alter the binding of S to E, which would affect Km
- affect E in ways to alter the actual catalysis of bound S, which would affect kcat
- affect E by globally changing the conformation of the protein
- affect S by altering the protonation state of the substrate
The easiest assumption is that certain side chains necessary for catalysis must be in the correct protonation state. Thus, some side chain, with an apparent pKa of around 6, must be deprotonated for optimal activity of trypsin which shows an increase in activity with the increase centered at pH 6. Which amino acid side chain would be a likely candidate?
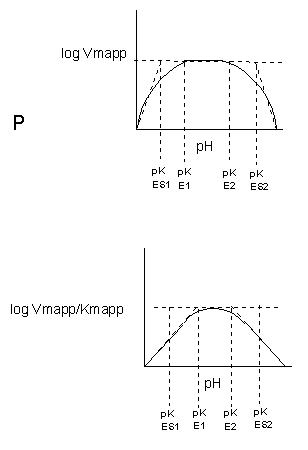
See the following figure which shows how pH effects on enzyme kinetics can be modeled at the chemical and mathematical level.
Figure: Chemical equations showing the mechanism of pH effects on enzyme catalyzed reactions
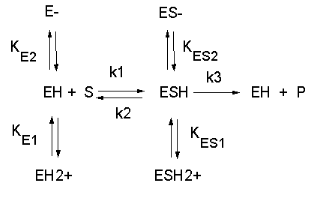
Figure: Mathematic equations modeling pH effects on enzyme catalyzed reactions
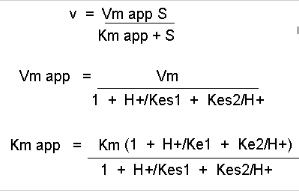
Figure: Graphs of pH effects on enzyme catalyzed reactions
Navigation
Return to Chapter 6C: Enzyme Inhibition Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 6C: Enzyme Inhibition

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.