ATP
Java version
HTML 5 version (does not require Java; downloads and moves
slowly)
The ATP molecule is the "universal carrier of free energy" in the biological world. Organisms use ATP
to drive biological oxidation reactions that are thermodynamically unfavored, such as protein and nucleic acid synthesis, and
transport against a concentration gradient. The charge density on ATP is larger than its hydrolysis product, ADP and theoretical studies show that ATP is
less hydrated than ADP. ATP is stable in solution if an enzyme catalyst is not present to drive hydrolysis or phosphoryl transfer.
For more information see
Biochemistry Online: Chapter 8B -
ATP and Oxidative Phosphorylation Reactions
Wire Frame
Phosphorus in Green
Carbons in Yellow
Hydrogens in Blue
Oxygens in Red
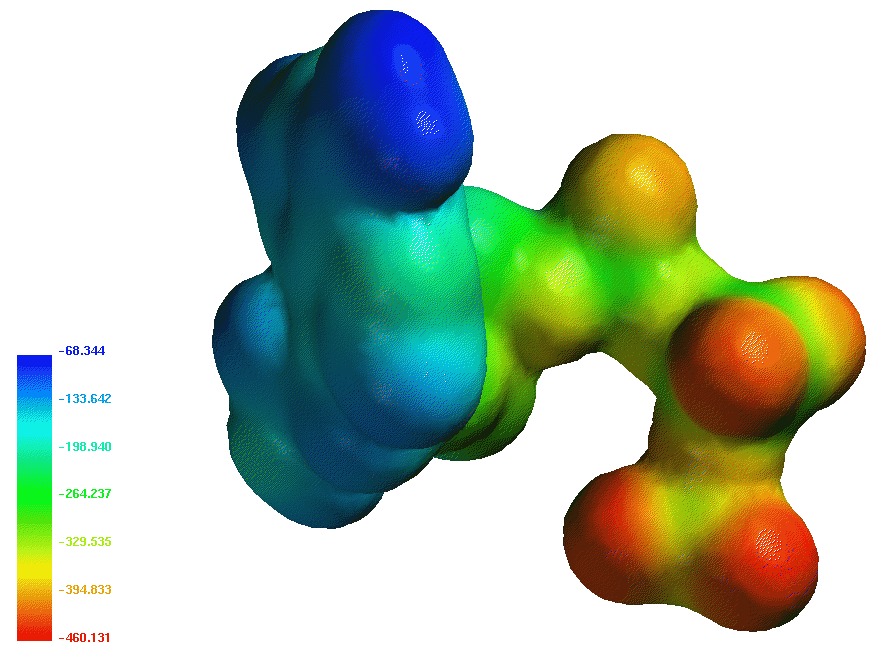
A map of the electrostatic potential (using Spartan) is shown above.