Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - METABOLIC AND SIGNAL TRANSDUCTION
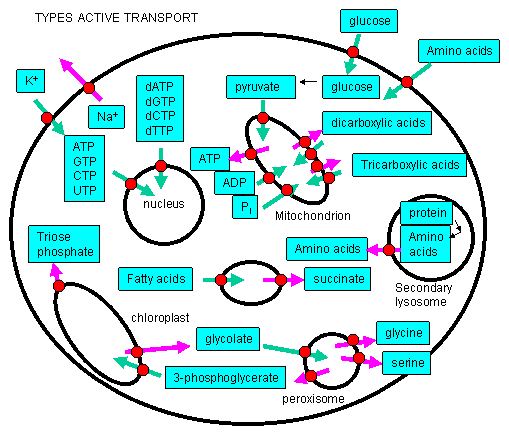
A. ACTIVE TRANSPORT
BIOCHEMISTRY - DR. JAKUBOWSKI
04/16/16
|
Learning Goals/Objectives for Chapter 9A:
|
A1. Introduction to Active Transport
We have previously discussed how chemical potential energy in the form of reduced organic molecules can be transduced into the chemical potential energy of ATP. This ATP can be used to drive reductive biosynthesis and movement (from individual cells to whole organisms). ATP has two other significant uses in the cell.
Active Transport: Molecules must often move across membranes against a concentration gradient - from low to high chemical potential - in a process characterized by a positive ΔG. As protons could be "pumped" across the inner mitochondrial membrane against a concentration gradient, powered by the ΔG associated with electron transport (passing electrons from NADH to dioxygen), other species can cross membranes against a concentration gradient - a process called active transport - if coupled to ATP hydrolysis or the collapse of another gradient. This active transport is differentiated from facilitated diffusion we studied earlier, which occurred down a concentration gradient across the membrane. Many such species must be transported into the cell or into intracellular organelles against a concentration gradient!
Signal Transduction: All cells must know how to respond to their environment. They must be able to divide, grow, secrete, synthesize, degrade, differentiate, cease growth, and even die when the appropriate signal is given. This signal invariably is a molecule which binds to a receptor, typically on the cell surface. (Exceptions include light transduction in retinal cells when the signal is a photon, and lipophilic hormones which pass through the membrane.) Binding is followed by shape changes in transmembrane protein receptors which effectively transmits the signal into the cytoplasm. We will discuss two main types of signal transduction pathways:
- nerve conduction, in which a presynaptic neuron releases a neurotransmitter causing a postsynaptic neuron to "fire";
- signaling at the cell surface which leads to activation of kinases within the cytoplasm;
We will discuss signal transduction in the final two chapters.
For active transport to occur, a membrane receptor is required which recognizes the ligand to be transported. Of major interest to us, however, is the energy source used to drive the transport against a concentration gradient. The biological world has adapted to use almost any source of energy available.
Energy released by oxidation: We have already encountered the active transport of protons driven by oxidative processes. In electron transport in respiring mitochondria, NADH is oxidized as it passes electrons to a series of mobile electron carriers (ubiquione, cytochrome C, and eventually dioxygen) using Complex 1, 3 and 4 in the inner membrane of the mitochondria. Somehow the energy lost in this thermodynamically favored process was coupled to conformational changes in the complex which caused protons to be ejected from the matrix into the inner membrane space. One can imagine a series of conformation-sensitive pKa changes in various side chains in the complexes which lead in concert to the vectorially discharge of protons.
ATP hydrolysis: One would expect that this ubiquitous carrier of free energy would by used to drive active transport. In fact, this is one of the predominant roles of ATP in the biological world. 70% of all ATP turnover in the brain is used for the creation and maintenance of a Na and K ion gradient across nerve cell membranes using the membrane protein Na+/K+ ATPase.
Light: Photosynthetic bacteria have a membrane protein called bacteriorhodopsin which contains retinal, a conjugated polyene derived from beta-carotene. It is analogous to the visual pigment protein rhodopsin in retinal cells. Absorption of light by the retinal induces a conformation changes in the retinal and protein, which leads to vectorial discharge of protons ;
Collapse of an ion gradient: The favorable collapse of an ion gradient can be used to drive the transport of a different species against a concentration gradient. We have already observed that collapse of a proton gradient across the inner mitochondria membrane (through FoF1ATPase) can drive the thermodynamically unfavored synthesis of ATP. Collapse of a proton gradient provides a proton-motive force which can drive the active transport of sugars. Likewise a sodium-motive force can drive active transport of metal ions. Since the energy to make the initial ion gradients usually comes from ATP hydrolysis, ATP indirectly powers the transport of the other species against a gradient.
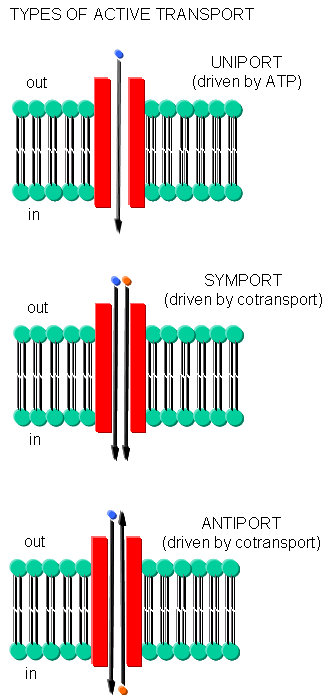
Often times, transport of one species is coupled to transport of another. If the species are charged, a net change in charge across the membrane may occur. Several terms are used to describe various types of transport:
- symport - two species are cotransported in the same direction by the same transport protein
- antiport - two species are cotransported in opposite directions by the same transport protein
- electrogenic - a net electrical imbalance is generated across the membrane by symport or antiport of charged species
- electroneutral - no net electrical imbalance is generated across the membrane by symport or antiport of charged species
A2. Transport of Ions
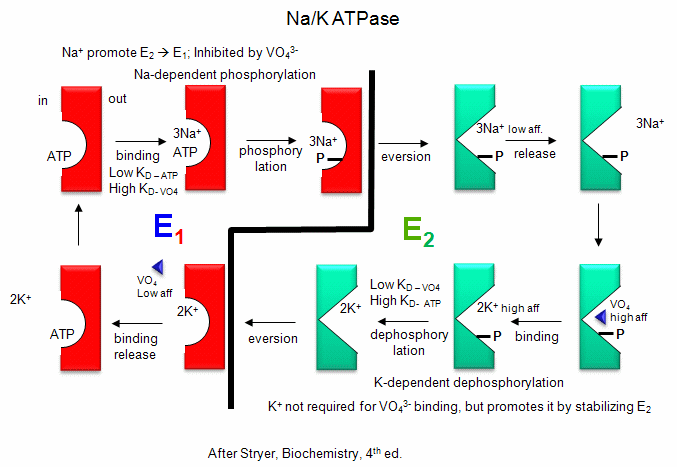
Na/K - These ions are both transported by the Na/K ATPase.
This protein keeps the K+in and Na+out high compared to their respective
concentrations on the other side of the membrane. The protein exists in two
essential conformations, E1 and E2, depending on the phosphorylation state
of the protein. ATP and 3 Na+ bind to the cytoplasmic domain of the enzyme
in the E1 conformation. In the presence of Na ions, the bound ATP is cleaved
in a nucleophilic atack by an Asp side chain of the protein. (Hence, the
protein is a Na+-activated ATPase. The phosphorylated enzyme changes
conformation to the E2 form in which Na+ ions are now on the outside of the
cell membrane, from which they dissociate. The phosphorylated protein in
conformation E2 now binds 2 K+ ions on the outside, which activates
hydrolysis of the Asp-PO3 mixed anhydride link. The dephosphorylated protein
is more stable in the E1 conformation to which it changes as it bring K+
ions into the cell. This is an example of an electrogenic antiporter.
Transport proteins that use this mechanism of transport are designated as P
types, since ATP cleavage is required and PO43- is covalenty transferred to
an Asp residue from the ATP. P-Type transporters are inhibited
by vanadate (VO43-), a transition state analog of phosphate. Note:
Transport mediated by P type membrane proteins can, in the lab, be used to
drive ATP synthesis.
Detailed kinetic analysis of ATP
and vanadate interactions show there are a low affinity and high affinity
site for each on Na/K ATPase. The high affinity vandate site appears
to be the same as the low affinity ATP site, which suggest that vandate
binds tightly to the E2 form of the enzyme. The low affinity vandate
site appears to be the same site (based on competition assays) as the ATP
site, which is probably the E1 form. Hence vandate binds
preferentially to the E2 form would inhibit the transition to the E1 form.
Vanadate also inhibits phosphatases, enymes that cleaves phosphorylated Ser,
Thr, and Tyr - phosphoesters in proteins. This supports the notion
that vanadate binds preferentially to the E2 form, which has a
phosphoanhydride link (Asp-O-phosphate) that is hydrolyzed, promoting the
conversion of E2 back to E1. Vanadate is probably at
transition state analog inhibitor in that it can readily adopt a trigonal
bipyramidal structure, mimicking the transition state for cleavage of the
tetrahedral anhydride bonds of ATP and Asp-O-PO4.
animation:
Na/K ATPase
Figure: Na/K-ATPase
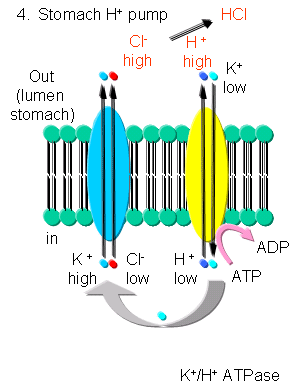
K - In addition to the above mechanism, K ions can be transported with protons in an electroneutral antiport mechanism by a K+/H+-ATPase found in stomach cells, which gives rise to a low pH in the lumen of the stomach.
Figure: K/H ATPase
Ca - Calcium levels are very low in cells. Transient increases are more
likely to be detected in a signal transduction pathways than a transient
decrease in high basal or constituitive cytoplasmic levels. Ca2+-ATPase,
homologous to the Na/K-ATPase, removes Ca2+ from the cytoplasm to either the
outside of the cells or into internal organelles. In addition a Na+-Ca2+
exchange protein (an antiporter) transports calcium ions out of the cell
using a sodium-motive potential.
Transport of calcium ions
Figure: Transport of calcium ions
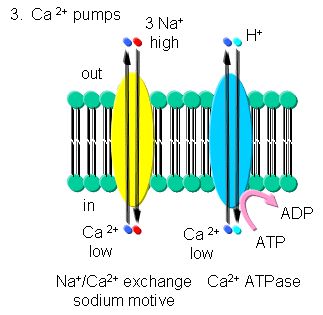
All of above ATPases are examples of P-type ion transporters.
A3. Transport of Sugars
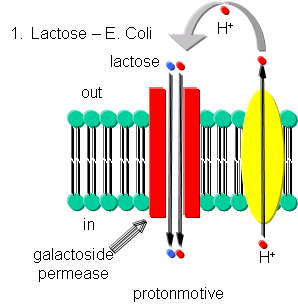
Lactose - Lactose can be transported into E. Coli against a concentration gradient using galactoside permease, one of the proteins encoded by the lac operon. This protein uses a proton-motive force to pump lactose into the cell. The proton gradient is created by an electron transport complex in the membrane which is inhibited by cyanide, reminiscent of the cytochrome C oxidase complex in oxphos.
Figure: Lactose Transport
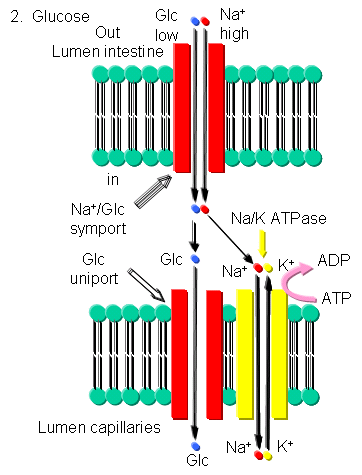
Glucose - Glucose can be transported into brush border cells lining the small intestine powered by a sodium-motive symport transporter.
Figure: Glucose Transport
A4. Transport of Protons
Driven by oxidation - The proton gradient formed during aerobic oxidation and photosynthesis in mitochondria and chloroplast, respectively, is paid for by free energy decreases associated with oxidation of organic molecules.
Driven by ATP cleavage - As mentioned above, protons are transported into the the lumen of the stomach by a K+-H+ ATPase.
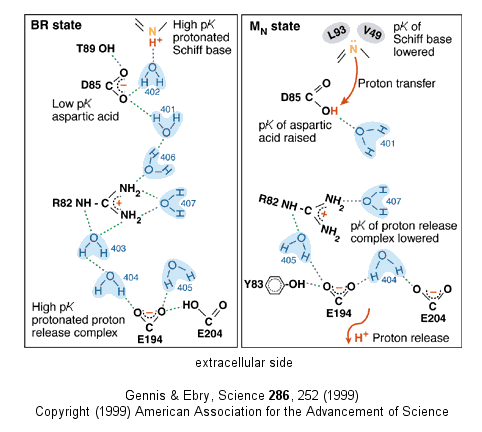
Driven by light - Photosynthetic bacteria have a membrane protein called bacteriorhodopsin which contains retinal, a conjugated polyene derived from beta-carotene. The retinal is covalently attached to the protein through a Schiff base linkage to an epsilon amino group of Lys (much as pyridoxal phosphate is in PLP-dependent enzymes). Bacteriorhodopsin is analogous to the visual pigment protein rhodopsin in retinal cells. Absorption of light by the retinal induces a conformational changes in the all trans-retinal, which causes an associated conformational change in bacteriorhodopsin. The initial state (BR) changes through a series of intermediates (K, L, M, N, and O). Various side chains and the protonated N of the Schiff base of retinal change their relative positions with respect to each other, which leads to changes in protonation states of the side chains and ultimately vectorial discharge of protons through the membrane. As the M state forms, H+ is moved to the extracellular side of the membrane (as shown below). Later a H+ is taken up on the cytoplasmic side (at the Schiff base of the retinal link) leading to reformation of the BR state. Experiments have been done to trap the protein in some of these intermediate states. In one (Leuke et al, 1999), a mutant (Asp 96 to Asparagine or D96N) trappped the protein in a state, MN, that occurs after a H+ has been moved to the extracellular side but before a compensatory H+ has been taken up on the cytoplasmic face. The mutation hinders the reuptake of the proton.
![]() Animation
of bacteriorhodopsin
Animation
of bacteriorhodopsin
Figure: BACTERIORHODOPSIN AND PROTON TRANSPORT
Figure: A NEW VERSION SHOWING
PROTON TRANSFER IN BACTERIORHODOPSIN
![]() Jmol:
Updated Bacteriorhodopsin Crystallized From Bicelles
Jmol14 (Java) |
JSMol (HTML5)
Jmol:
Updated Bacteriorhodopsin Crystallized From Bicelles
Jmol14 (Java) |
JSMol (HTML5)
A5. Other ATP Powered Transporters
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) - This is a member of a family of an ATP-Binding Cassette or ABC transporter proteins. The membrane protein has 12 transmembrane helices. In contrast to other ion transporters which transport a discrete number of ions (3 sodium and 2 potassium ions, for example), this changes conformation to form an open pore through which chloride ions flow. This protein is defective in Cystic Fibrosis.
Multidrug Resistance Transporter - MDR - This is another example of an ATP-Binding Cassette or ABC transporter. It acts in a somewhat promiscuous fashion in pumping nonpolar toxic molecules out of the cell. This would seem quite beneficial to the organism, unless the toxic molecule is a chemotherapeutic drug used to kill a tumor cell.
![]() Molecular
Dynamics simulation of MDR protein in membrane
Molecular
Dynamics simulation of MDR protein in membrane
![]() Jmol
: Updated EmrE multidrug resistance transporter
Jmol14 (Java) |
JSMol (HTML5)
Jmol
: Updated EmrE multidrug resistance transporter
Jmol14 (Java) |
JSMol (HTML5)
Phospholipid Flippase or Transbilayer amphipath transporter (TAT) - This is a member of the P-Type ATPase family which instead of moving ions across the membrane flips amino lipids (like PE) across leaflets in the bilayer. In an early chapter we noted that flip-flop diffusion in liposomes was slow compared to that in cells, suggesting that the flip-flop diffusion was catalyzed in the cell. Catalysis requires ATP cleavage and produces two conformations of the protein. During the conformational change of the protein, a phospholipid appears to bind to the protein and is flipped to the other side of the membrane.
The disposition of phosphatidylserine, a
negatively charged phospholipid, between membrane leaflets is
especially interesting and important. Almost all the PS is localized
in the inner leaflet. Cells in which PS is found in the outer leaflet
are target for program cell death (apoptosis). PS in the outer leaflet
can also promote blood clotting as clotting factors are recruited to the
surface. It appears that a P-type ATPase is required. Using gene
silencing by RNA interference in C. Elegans, Darland-Ranson found that
onespecific P-type ATPase, TAT-1 out of 6 found in the organisms had PS
flippase activity, which would retain PS in the inner leaflet. Cells
with PS in the outer leaflet were often targets of phagocytosis, suggesting
the phagocytes have receptors that recognize PS. Cells with PS
receptors may also bind and internalize virus, which have membrane leaflets
acquired from infected cells as the virus buds off from the cells.
Such cells might have PS in their outer leaflets since the infected cells
may be in the process of dying through apoptosis, which would increase PS in
the outer leaflet.
![]() Experimental
Study of Flipping:
Labeling new PL ;
Assay for Flip-flop Diffusion
Experimental
Study of Flipping:
Labeling new PL ;
Assay for Flip-flop Diffusion
There are also other types. F-type are similar to the F0F1ATPases and can transport protons against a concentration gradient powered by ATP breakdown. Notice that this is the opposite role for this enzyme that we discussed in mitochondrial oxidative phosphorylation. V-type (vacuolar) are found in the membranes of acidic organelles (like lysosomes) and acidic vesicles within neurons, where neurotransmitters are stored.
As mentioned earlier, one of the biggest problems in medical drug development is the productions of drugs that can diffuse across the cell membrane. This requires that the drug be sufficiently nonpolar while at the same time being sufficiently polar to have reasonable aqueous solubility, allowing blood transport. Another approach to getting drugs across the membrane is to modify them to bind to transporters whose normal function is to move solutes against a concentration gradient across a lipid bilyaer. The extent of modification of the drug depends on how close the structure of the drug is in comparison to the normal ligand for the transporter. This approach has been used by the biotech company XenoPort, to develop drugs that can be more readily absorbed by the small intestine, which has many active transporters designed to move nutrients into cells.
A6. Links and References
-
Science 320: 528 (2008)
- The xyz of ABC Transporters (require ATP, overexpression lead to resistance to antibiotics, chemotherapy, etc). Science. 293, pg 1782 (2001)
- Bacteriorhodopsin- the movie. Nature. 406, pg 569, 645, 649, 653 (2000)
- Toyoshima et al. Structure of sacroplasmic reticulum Ca pump. Nature. 405, pg 633, 647 (2000)
- Luecke, H. et al. Structural Changes in Bacteriorhodopsin During Ion Transport at 2 Angstrom Resolution, Science 286, 255 (1999).

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.