Reactivity in Chemistry
Electrophilic Aromatic Substitution
AR6. Side Chain Oxidation
The introduction of a side group on a benzene ring is just one aspect of benzene reactivity. Often, these groups undergo unique reactions that can be synthetically useful. These reactions are sometimes aided by by the special position next to the aromatic ring; that position can lead to stabilization of reactive intermediates.
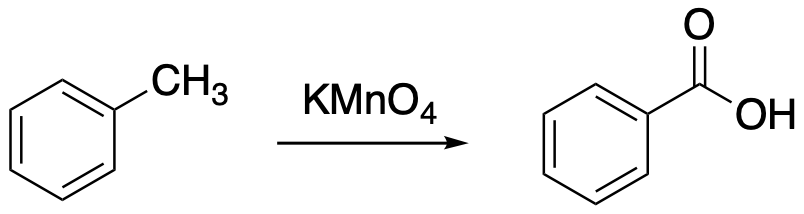
An oxidation reaction in organic chemistry usually results in the formation of new C-O bonds. In this section, we will look at an oxidation reaction in which oxygen atoms are introduced on a carbon at the position next to the aromatic ring. Remember, that carbon directly attached to the benzene is called the benzylic position. The name, benzyl chloride, means C6H5CH2Cl, with the chloride attached to the benzylic position; the name, benzoic acid, means C6H5CO2H, with the carboxylic acid carbon attached to the benzene ring. The introduction of oxygen atoms always leads to the formation of a carboxylic acid in these cases.

Figure AR6.1. Oxidation of the benzylic position with permanganate.
The replacement of a hydrogen atom by an oxygen atom is considered an oxidation. In oxidation state formalisms, a hydrogen is considered to have a 1+ charge, whereas an oxygen is considered to have a 2- charge. To balance that charge, an atom attached to hydrogen would have a more negative oxidation state (or a less positive one) if attached to hydrogen but a more positive oxidation state (or a less negative one) if attached to oxygen.
If we are replacing oxygen with hydrogen, we need a source of oxygen atoms. If we are oxidizing the benzylic position, that means we are reducing something else. Oxidation-reduction reactions always involve the transfer of electrons from one place to another. If one thing is getting oxidized, losing electrons, then something else is getting reduced, gaining electrons. This combination is most often supplied in the form of potassium permanganate, KMnO4. The permanganate ion contains a high oxidation state Mn(VII); formally, the manganese has lost all of its valence electrons to the attached oxygens, which are more electronegative than the manganese. The manganese could accept two electrons to become Mn(V); it might even accept four electrons to become Mn(III). This concept is similar to the one behind oxidation of alcohols.
A key part of this reaction is that the oxygen has to replace at least one hydrogen atom at that position. There can also be carbons at this position, but there has to be at least one hydrogen there for the reaction to work. So, the reaction will work with methylbenzene or toluene, leading to benzoic acid. It also works with ethylbenzene, and benzoic acid is again the product in that case. However, the reaction will not proceed in the case of tert-butylbenzene.
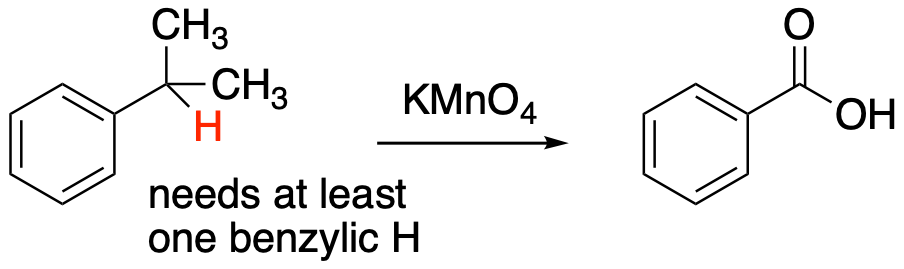
Figure AR6.2. A benzylic hydrogen is required for benzylic oxidation.
This fact is just one of the reasons chemists long suspected a role of radicals in the reaction. Radicals are unstable intermediates in which an atom has an unpaired electron. For example, a carbon radical might have three bonds and a single, unpaired electron. It has no formal charge, but it is still electron-deficient, lacking an octet. Like other reactive intermediates, radicals can be stabilized by certain factors; in this case, the most important factor is resonance delocalization. A radical next to a benzene ring can be delocalized by resonance. If this reaction involves a radical, it would make sense that it happens selectively at the benzylic position. The role of radicals in benzylic oxidation was experimentally confirmed by the lab of James Mayer, now at Yale University, during the 1990's. The subsequent steps leading to the carboxylic acid are more complicated.
Problem AR6.1.
Use resonance structures to show why removal of a hydrogen atom from methylbenzene results in a stable radical.
Problem AR6.2.
Predict the products if each of the following compounds were treated with potassium permanganate.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted. It is freely available for educational use.

Structure & Reactivity in Organic,
Biological and Inorganic Chemistry by
Chris Schaller
is licensed under a
Creative Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Navigation: