CO14. Addition of Neutral Nucleophiles
Nucleophiles do not have to be ionic, or even "semi-anionic". The basic requirement for a nucleophile is a lone pair. If a nucleophile has a lone pair, it can donate the lone pair to an electrophile such as a carbonyl. By donating a lone pair to a carbonyl, it can form a bond.
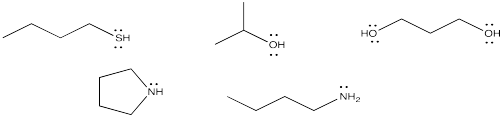
Figure CO14.1. Some neutral nucleophiles.
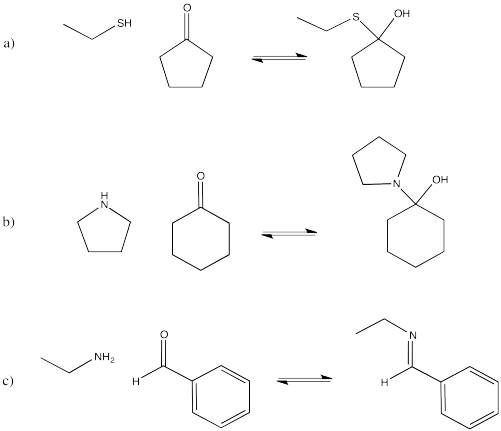
However, donation of a lone pair to a carbonyl is reversible. If there is something about the nucleophile/electrophile adduct that isn't very stable, the reaction may revert to reactants again. In the case of neutral nucleophiles, charge separation may destabilize the first-formed products of the reaction. Let's think about addition of water to propanone. Two neutral molecules, water and propanone, come together. The water donates a lone pair to the carbonyl carbon in propanone. That leaves the oxygen atom from the water with a positive charge, and the oxygen atom from the propanone with a negative charge.

Figure CO14.2. Addition of water to a carbonyl.
One easy way to get rid of the charge separation is for the water to leave again. That step would just be the reverse of the first one.

Figure CO14.3. Elimination of water from a carbonyl:water adduct.
On the other hand, another way to solve the charge problem is to move a proton (H+) from the positively charged oxygen to the negatively charged one. That turns out to be pretty easy to do. If that happens, a "hydrate" or a "geminal diol" forms. A geminal diol, or twin diol, has two hydroxy groups on one carbon.
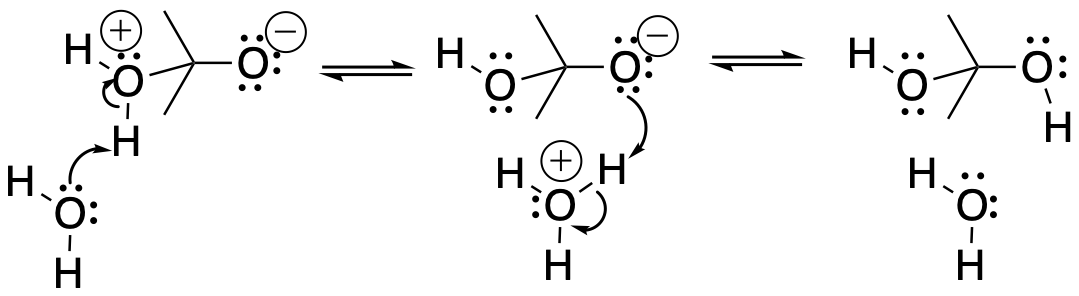
Figure CO14.4. Formation of a carbonyl hydrate or geminal diol.
- Nucleophiles don't have to be ions; they just need a lone pair.
- Neutral nucleophiles generally have a proton attached to the nucleophilic atom.
- This proton is lost after the nucelopile donates to the electrophile.
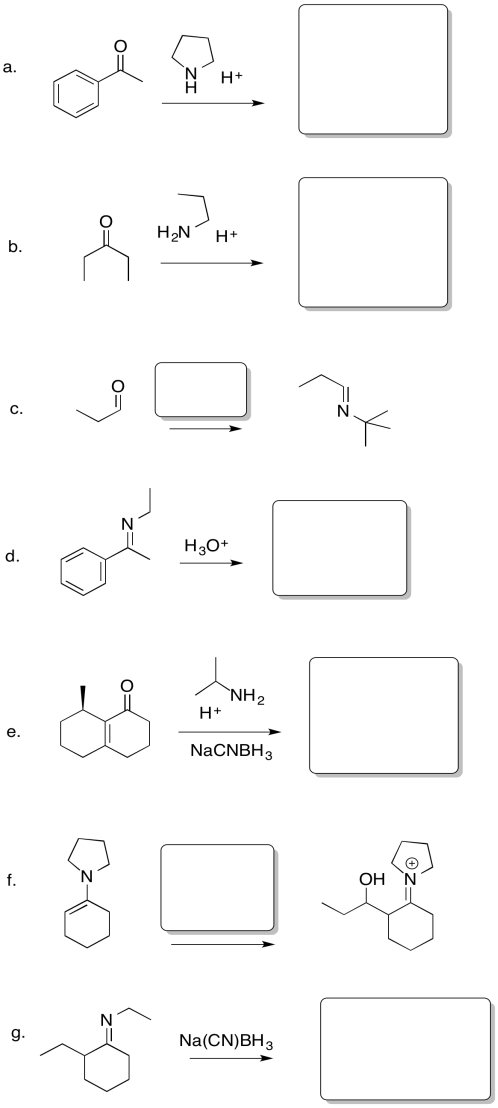
ProblemCO14.1.
In the following cases, a nucleophile donates to the carbonyl, followed by a proton transfer. Show mechanisms, with curved arrows, for each of the following reactions.
The immediate products that form from the addition of neutral nucleophiles to carbonyls turn out to be a little unstable. That's partly because they can easily revert back to reactants. It's also because there are other processes that carry the reaction away from the first-formed products and turn them into other things. For example, in most cases the reaction does not involve the simple addition of one nucleophile, but involves a second molecule of the nucleophile as well.
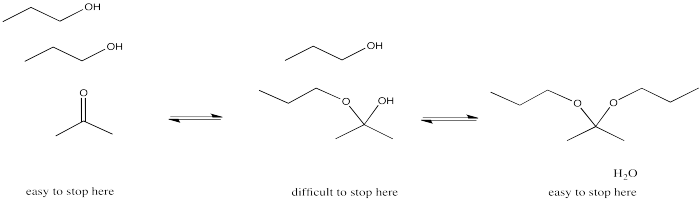
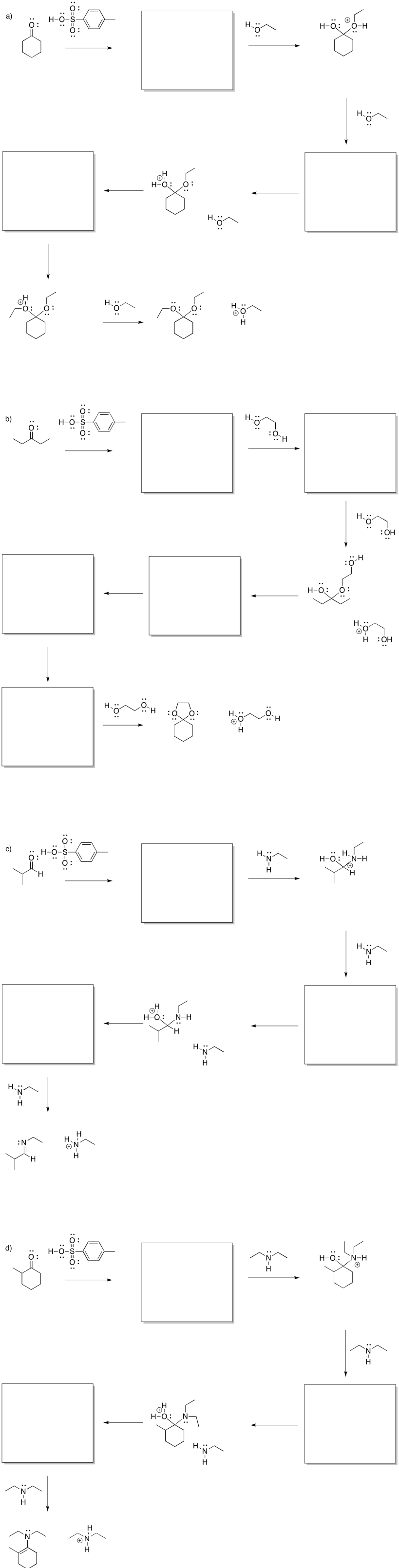
Figure CO14.5. Formation of an acetal or ketal.
We will keep looking at these other processes and see where these reactions lead. In the meantime, it can be useful to know the patterns that different types of nucleophiles will usually follow. For example, addition of alcohols to aldehydes or ketones leads to the formation of ketals or acetals. Ketals or acetals have the specific chain of atoms C-O-C-O-C, in which each carbon is tetrahedral or sp3. The two terms refer to alcohol adducts of ketones, which are ketals, or the alcohol adducts of aldehydes, which are acetals. The word acetal is often used generally to mean either one.
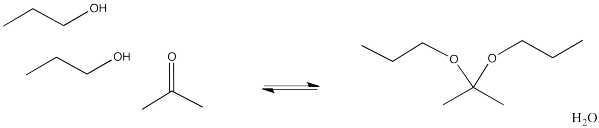
Figure CO14.6. The overall reaction for acetal or ketal formation.
-
Addition of a neutral nucleophile to a carbonyl almost always leads to loss of the original carbonyl oxygen in a water molecule.
-
With alcohols, two oxygen groups replace the carbonyl oxygen.
So, in general, two alcohol nucleophiles add to an aldehyde or ketone. The carbonyl oxygen is displaced as a water molecule. Formally, the protons in the water molecule come from the OH groups on the alcohol nucleophiles. The acetal product forms reversibly, which means the reaction could be driven back to the aldehyde or ketone by adding water to the acetal.
Two alcohols and an aldehyde make an acetal. WHat happens if the nucleophile has two hydroxy groups instead of just one? Then there is a possibility that both of the nucleophilic oxygens come from the same molecule. The two OH groups are already connected to each other in the reactant. They will also both be connected to the same carbon in the product. That means the product will contain a new ring. This is especially easy to do if the ring is 5- or 6-atoms around, because atoms in a chain can wrap around and connect with each other pretty easily if they are in the range of 5- or 6-atoms down the chain from each other.
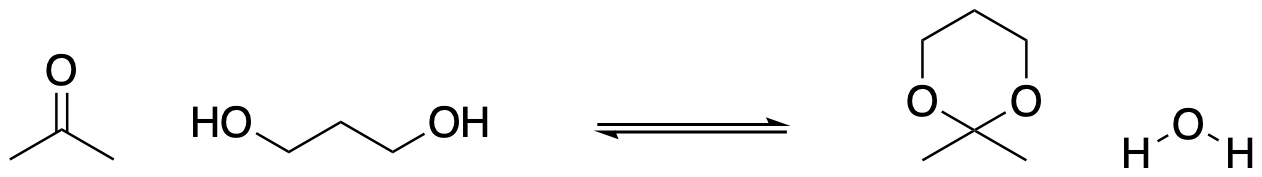
Figure CO14.7. Formation of a cyclic acetal.
Formation of cyclic acetals is even easier than forming acetals from two different alcohol molecules. That's true for a couple of different reasons. First of all, you can imagine that once one of the OH groups in a diol has bonded to the carbonyl compound, the second one becomes more likely to do the same thing, because it is being held relatively close to the electrophile. The diol already has a hold of the electrophile; it's easier to pull it in and make the second bond.
- A diol and a ketone or aldehyde can form a cyclic acetal.
- This is especially easy with 1,2-diols and 1,3-diols to make 5- and 6-membered rings.
Secondly, there is a big entropy advantage in cyclic acetal formation compared to a regular acetal formation. Formation of a regular acetal is actually disfavourable by entropy, which pushes the equilibrium back toward reactants. Formation of a cyclic acetal is entropy-neutral, so the reaction doesn't have that built-in disadvantage. Enthalpically, the two versions are about the same: in both cases we are trading in a C=O double bond for two C-O single bonds. So the entropy difference is especially important here. Most of the time, entropy changes in reactions are pretty small, so you don't have to worry about it, but this time is different.
Why is there a big entropy difference in one reaction but not the other? Remember, a very simple way to assess entropy change in a reaction is just to count up the number of particles or molecules before the reaction and compare them to the number of molecules after the reaction. The more molecules, the greater the entropy. With two alcohol nucleophiles, the number of molecules before and after the reaction goes from 3 to 2. That's a decrease in the number of molecules. That's a decrease in entropy. That's not favourable. But with a diol, there are two molecules before the reaction: the diol and the carbonyl compound. There are two molecules after the reaction: the acetal and the water. So this reaction is entropically neutral. It isn't actually disfavoured like the regular acetal formation.
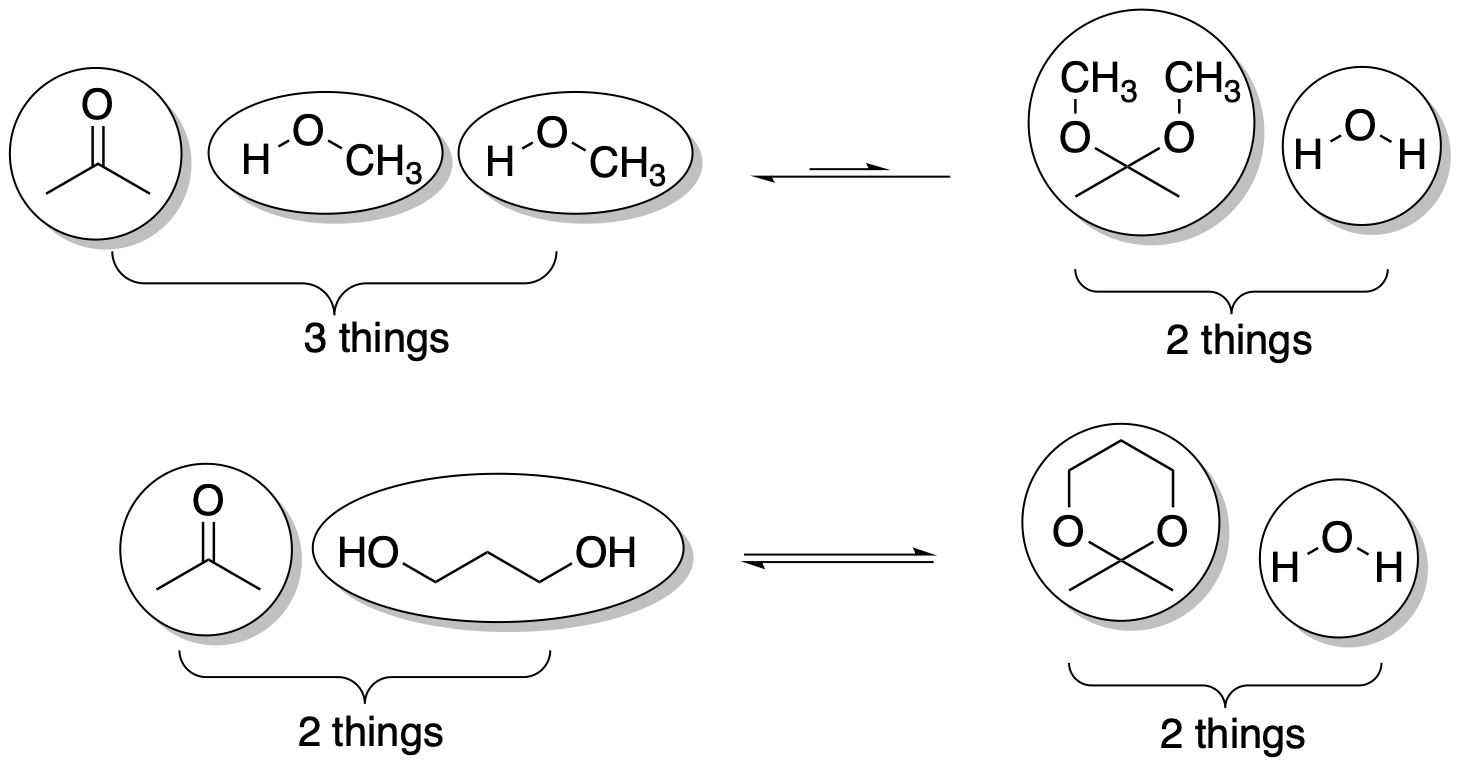
Figure CO14.8. Entropy changes in acetal formation.
- The formation of cyclic acetals is easier than the formation of non-cyclic acetals for entropy reasons.
On the other hand, the addition of amine nucleophiles to aldehyde or ketone electrophiles leads to a very different kind of structure than what we saw with alcohol nucleophiles. Instead of adding two amine molecules into the final structure, only one amine is incorporated, and a double bond appears again. The C=O of the aldehyde or ketone is replaced with the C=N of an imine. The product structure has a C=N-C or C=N-H pattern. Imines form important linkages in biological chemistry, especially in connecting small molecules to lysine side chains in proteins.
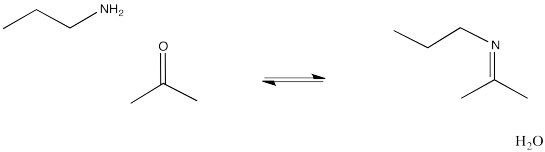
Figure CO14.9. Formation of an imine.
If the amine is "secondary", meaning the nitrogen is connected to two carbons instead of just one, the double bond ends up one position over. It is between the former carbonyl carbon and the one next to it, which is called the alpha position. This structure has a pattern C=C-NR2. This functional group is called an enamine, like ene-amine, because there is a carbon-carbon double bond (alkene) next to a nitrogen group (amine). Enamines, like imines, can play a role in biological reactions.
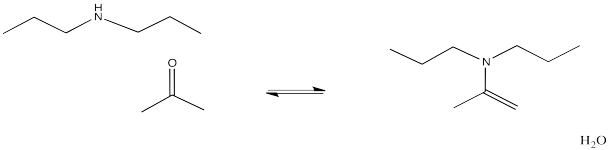
Figure CO14.10. Formation of an enamine.
-
With amines, one nitrogen group replaces the carbonyl oxygen, and a double bond forms.
- With primary amines, an imine is formed, containing a C=NR group.
- With secondary amines, an enamine is formed, containing a C=C-NR2 group.
Note that all of these reactions involve ultimate loss of water from the original carbonyl compound. That's a very important feature of reactions with neutral nucleophiles.
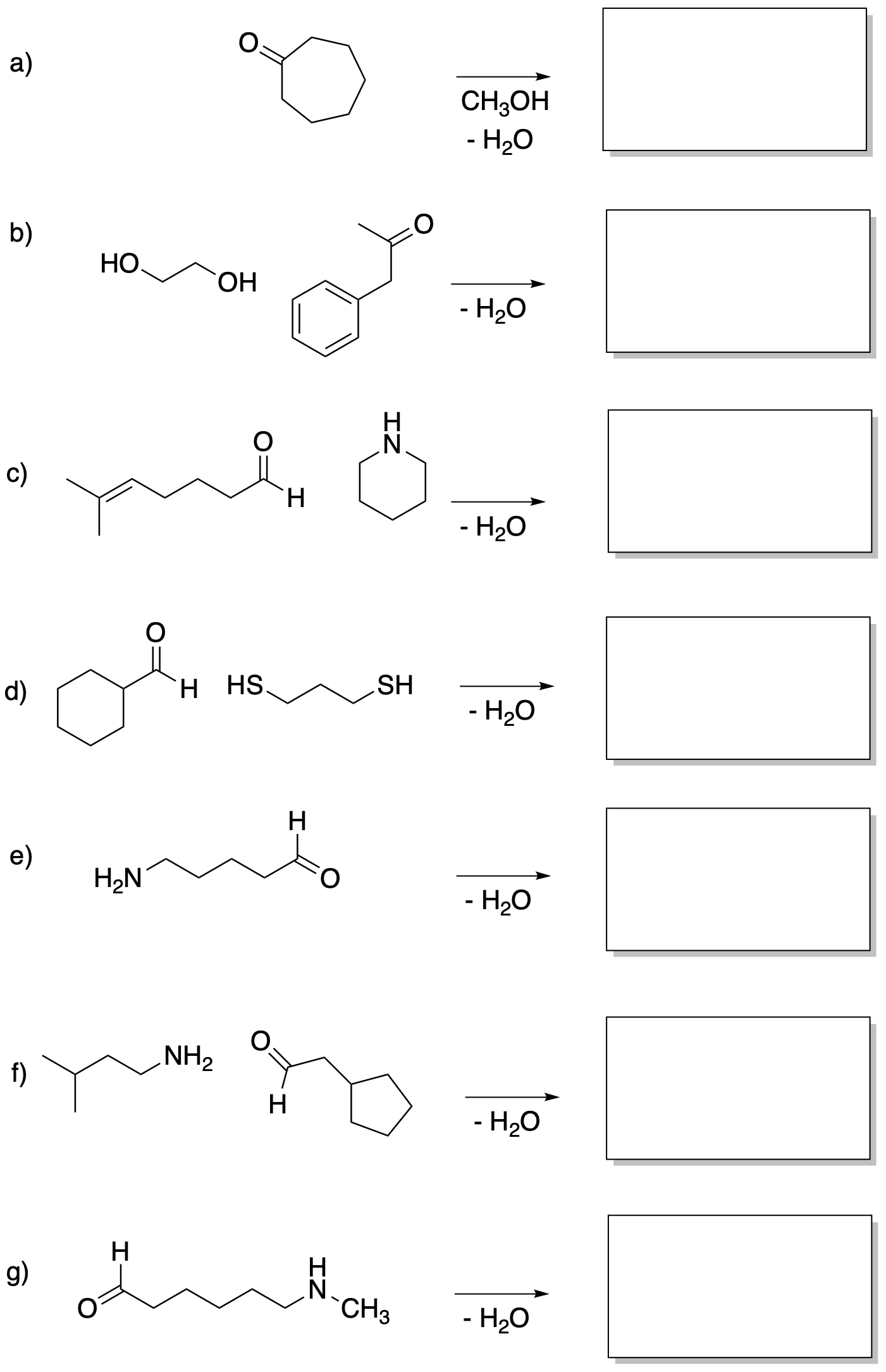
Problem CO14.2.
Fill in the missing reagents or products in the following reaction equations.