Reactivity in Chemistry
Substitution at Carboxyloids
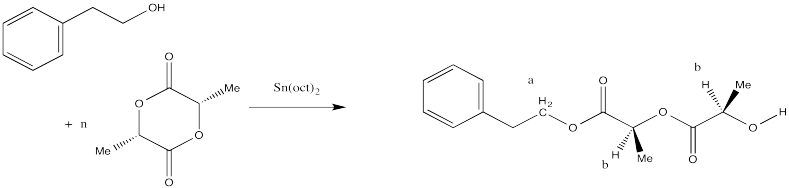
CX8b. Ring-Opening Trans-Esterification Polymerization
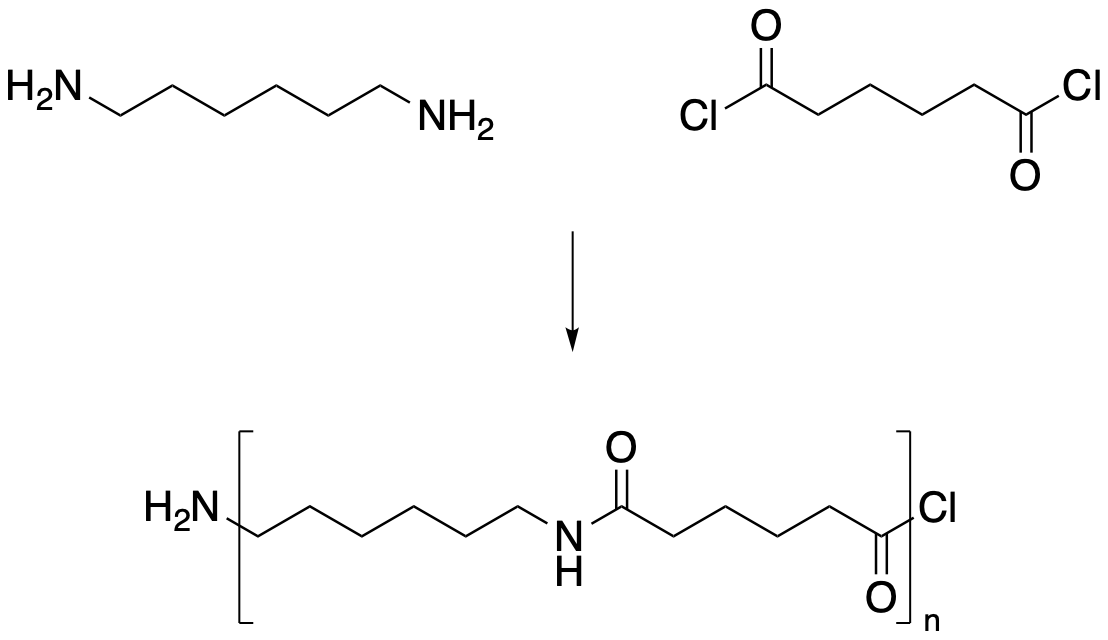
Difunctional molecules can sometimes polymerise, provided they have appropriate partners with which they can react on other molecules. For example, hydroxyesters might react with other hydoxyesters, with the hydroxyl group on one molecule reacting with the ester group on another, forming a polyester. Alternatively, there might be two different kinds of molecules that react with each other. For example, a diamine might react with a diacid chloride to form an amide.

Figure CX8b.1. Formation of a polyamide or nylon.
In ring-opening polymerisation, the monomer is not difunctional. Instead, it is embedded in a ring. Ideally, there is a little ring strain in the molecule, bumping it up in energy just a little so that it will react more easily. Common examples include caprolactone and lactide, used to make biodegradable yard waste bags and produce containers, respectively (among many other applications). These cyclic esters are sometimes referred to as "lactones".
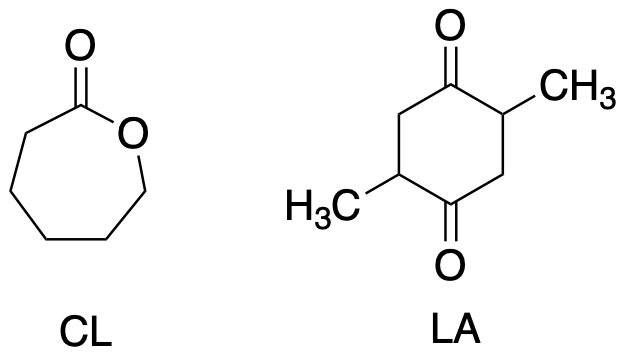
Figure CX8b.2. Two lactones: caprolactone (CL) and lactide (LA).
If an alcohol is added, it can act as an "initiator" in a "chain reaction". The alcohol is a nucleophile, and it donates to the carbonyl, eventually cleaving the carboxyl C-O bond and popping open the ring.
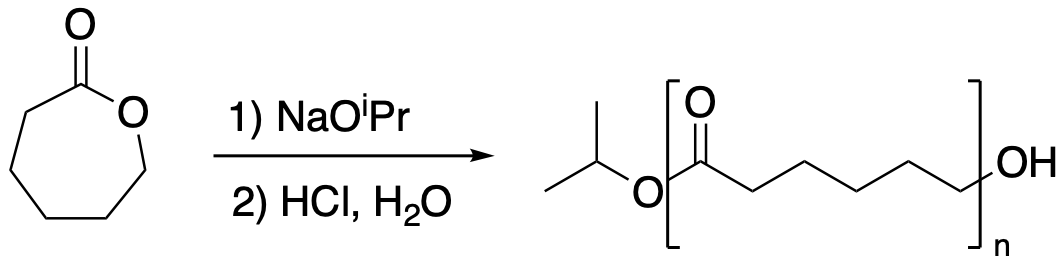
Figure CX8b.3. Formation of a polyester from a lactone.
At some point, a proton gets transferred to the oxygen that used to be embedded in the ring. Now we have a new alcohol. What does it do? It reacts with another cyclic ester, popping it open and forming a new alcohol. The cycle repeats itself.
This is an example of a chain reaction. Chain reactions are self-sustaining: once the reaction starts, it keeps on going. That is often because the product of the reaction is able to go back and start the reaction one more time.
In reality, ring opening polymerisations don't really work if you just add an alcohol to a lactone. Typically, a catalyst is also added. Catalysts most commonly are Lewis acids, such as aluminum, iron or tin compounds. One of the most common catalysts is tin octoate, more properly called tin(II) 2-ethylhexanoate.
Problem CX8b.1.
Provide a mechanism, with arrows, for the ring-opening polymerisation of caprolactone with tin octoate.
Problems CX8b.2.
Perform end-group analysis in the following cases to determine
a) the degree of polymerisation (what is the value of "n"?).
b) the molecular weight.
i. The ratio of the integrals for the 1H NMR peaks representing positions b:a is 50:1.
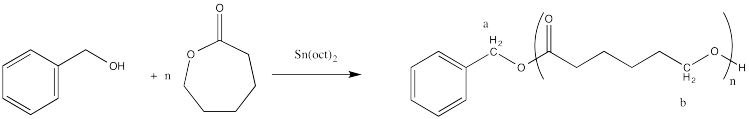
ii. The ratio of the integrals for the 1H NMR peaks representing positions b:a is 80:1.
End Group Analysis
These polyesters contain two different end groups. The initiator, a nucleophilic alcohol, becomes one of the end groups when it ring-opens the first monomer. The other end group is formed after polymerization when the reaction is normally quenched by adding some dilute acid such as HCl in methanol. Instead of being attached to another carbonyl, the very last oxygen in the chain is attached to a hydrogen. The last functional group is therefore an alcohol rather than an ester, so it is different from all of the other analogous positions along the chain.
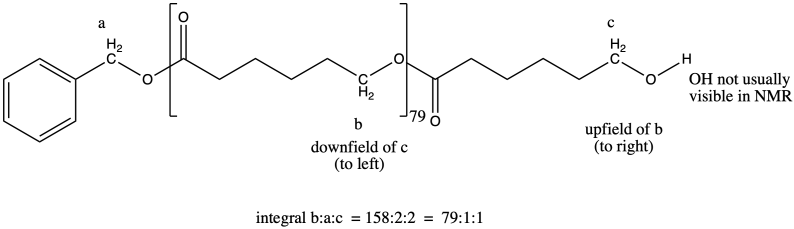
Figure CX8b.4. Possible end groups in a caprolactone sample.
It is tempting to think of that last proton, or the last OH group, as the second end group in one of these polymers. Strictly speaking, that is correct, but if we are using NMR for end group analysis then thinking about it that way presents a problem. More often than not, hydroxyl protons are not observed in the NMR spectrum, because they can exchange too quickly with other OH groups in water or other molecules. It is very easy for NMR solvents to become damp over time, so this problem is pretty common.
Normally when we are looking for an alcohol in an NMR spectrum, we don't look for the O-H proton. We look for the C-H proton attached to the hydroxyl group. It's usually easy to find because it is at a pretty unique are of the spectrum (3-4 ppm). (O-H protons, even if you can see them in the NMR, have relative freedom to move around in the spectrum based on additional factors such as concentration, which influences the extent of hydrogen bonding.) Furthermore, its shift would be slightly different from the shift of a similar C-H proton that was part of an ester; in most cases, we would expect the peak in the ester to be slightly downfield because of the adjacent carbonyl. As a result, there are always two different end groups in these ring-opened polymers, and if we can't find one, we should be able to find the other one.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry