Reactivity in Chemistry
Substitution at Carboxyloids
CX4b. Bringing All The Steps Together: Ester and Amide Hydrolysis
The reaction mechanisms of carboxyloids require only a few basic steps. They can seem a little complicated because several of these steps may be required to get from one point to another. However, each of these steps is a logical requirement, rather than an arbitrary fact to memorize.
The hydrolysis of an ester is a good example of a mechanism that is built on a few basic steps. These hydrolysis reactions take place in water, but esters themselves are pretty stable to water. Remember, our cell membranes contain a lot of ester linkages, and we might get a little pruny if we spend too long in the water, but we don't fall to pieces. To break up an ester, we need to add a catalyst. Acids and bases are the simplest kinds of catalysts, so let's take a look at how and ester can be broken down into a carboxylic acid and an alcohol through the addition of hydrochloric acid.

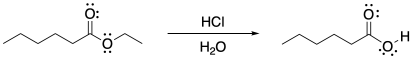
Figure CX5.1. Ester hydrolysis under acidic conditions.
Hydrochloric acid is an aqueous solution of hydrogen chloride. The hydrogen chloride molecules would have already transfered their protons to the water, so we often write HClaq as H3O+ Cl-. Both components of the solution, the hydrogen chloride and the water, take active roles as electrophile and nucleophile, respectively. The ester is not electrophilic enough to attract a water molecule as a nucleophile, so it must become activated by picking up a proton.
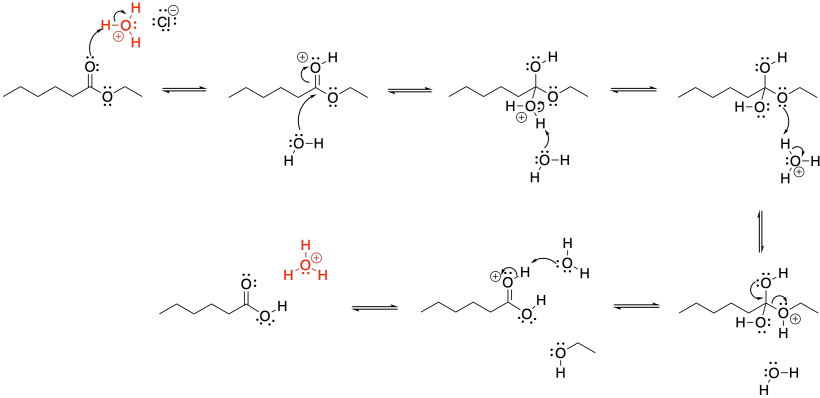
Figure CX5.1. The mechanism of ester hydrolysis under acidic conditions.
Once the protonated ester has lured in the water, the reaction has been partly accomplished, but the alkoxide must be induced to leave. To make it a better leaving group, the alkoxy oxygen is protonated. At that point, the entire alcohol group can be pushed out easily via π-donation.
Notice that, by the end of the mechanism, the acid has been regenerated again. It's ready to go and react with a second ester molecule, and then a second, a third, and so on. The regeneration of the species that got the reaction going is a hallmark of catalysis.
You may also have noticed that, rather than picking up the proton on that former water nucleophile with another water molecule, only to drop it off again on the alkoxy oxygen, we could take a shortcut. Why not just transfer the proton directly from the OH group to the OEt group? It's quicker and it's easier to draw. It's a good idea on paper, but it doesn't work that way. The OEt oxygen simply can't reach the OH2 proton. You can review that idea in the section on conformational analysis. So, we need the water to act as a proton shuttle between one site and the other.
Problem CX4b.1.
Draw the mechanism, with intermediates and curved arrows, for the hydrolysis of an amide under acidic conditions.
Ester hydrolysis can also be accomplished in base. This reaction is sometimes called the "saponification" of esters (literally "turning esters into soap"). In the old days, soap would be made by taking animal fats and mixing them with lye, breaking the ester linkages in the fats to make carboxylate salts.
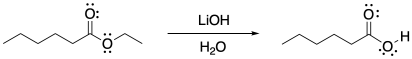
Figure CX5.3. Ester hydrolysis under basic conditions.
Under these conditions, instead of trying to convert the ester into a better electrophile to draw in the sluggish water nucleophile, we are taking the opposite approach. We are using a better nucleophile to react more rapidly with the reluctant ester electrophile. The mechanism still involves some very basic steps: nucleophilic addition and π-donation.
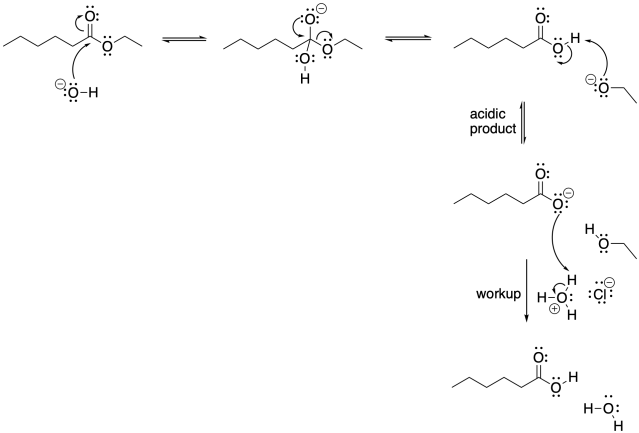
Figure CX5.4. The mechanism of ester hydrolysis under basic conditions.
Under these conditions, the end of the reaction seems a little more complicated than it has to be. What's going on? Remember that the product of tis reaction is a carboxylic acid. Under basic conditions, the carboxylic acid will lose its proton. To put the proton back, we would have to perform an acidic workup, neutralizing the base.
Problem CX4b.1.
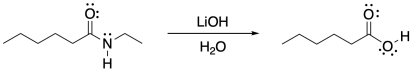
Draw the mechanism, with intermediates and curved arrows, for the hydrolysis of an amide under basic conditions.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry