Reactivity in Chemistry
Substitution at Carboxyloids
CX11. Additional Problems
Problem CX12.1.
FIll in the blanks in the following syntheses.

Problem CX12.2.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl and nucleophilic substitution at carboxyl.
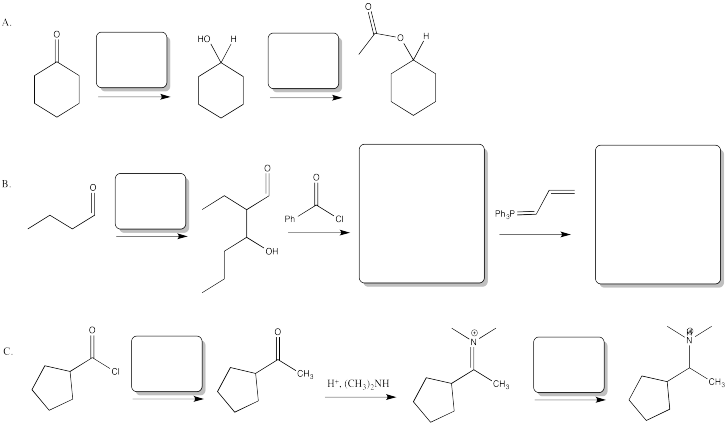
Problem CX12.3.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl and nucleophilic substitution at carboxyl.
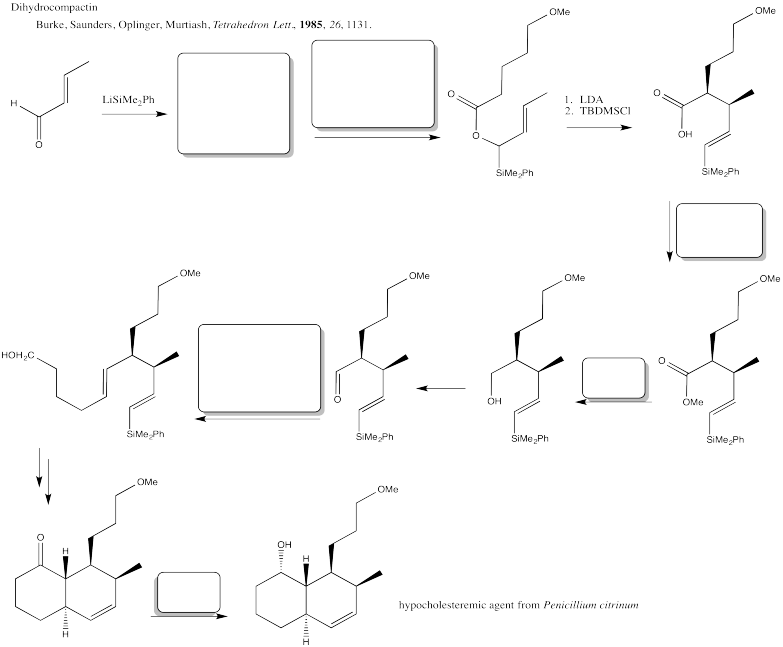
Problem CX12.4.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl and nucleophilic substitution at carboxyl (including Wittig and aldol reactions).
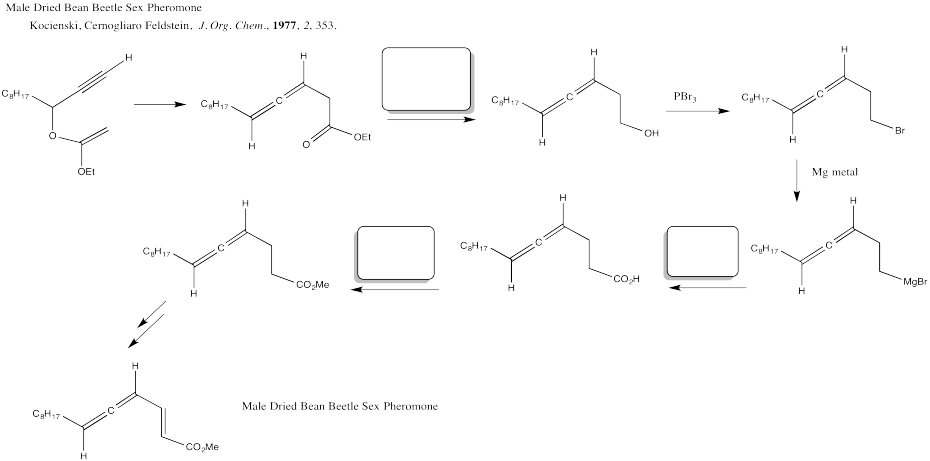
Problem CX12.5.
Fill in the blanks in the following synthesis involving nucleophilic substitution at carboxyl.
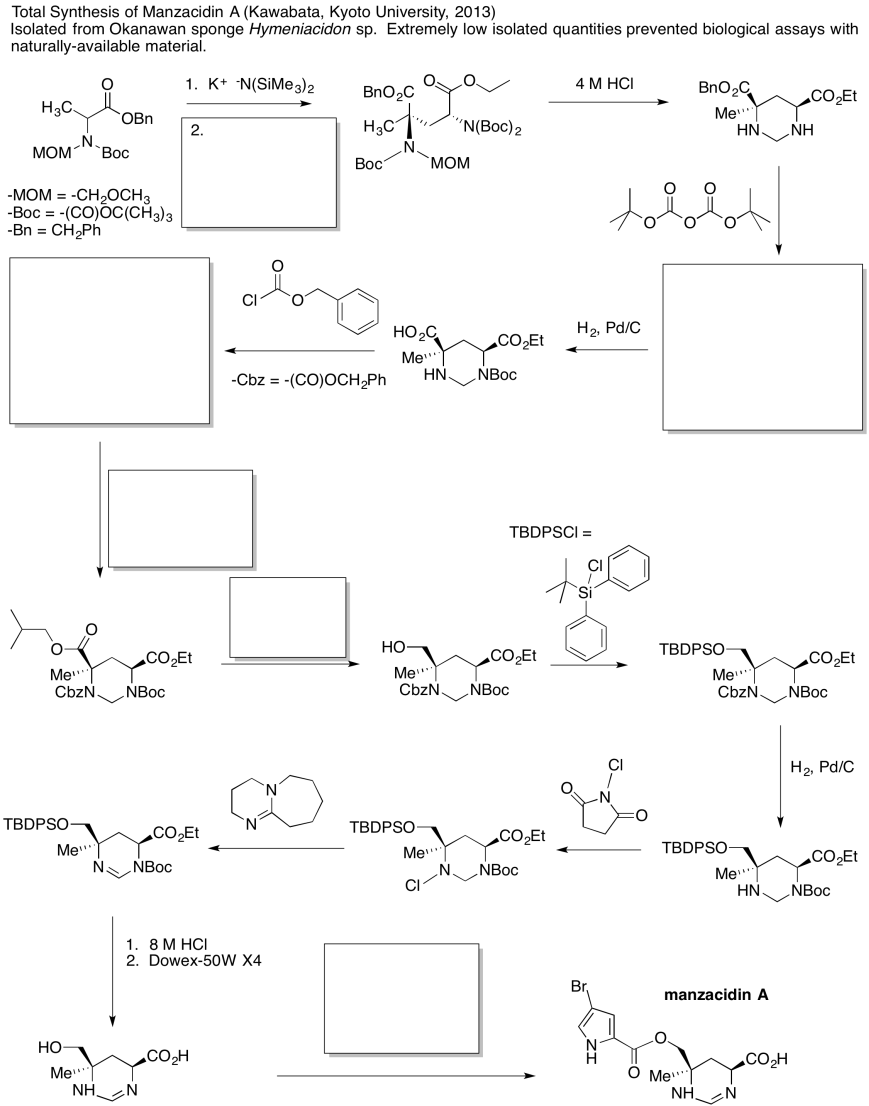
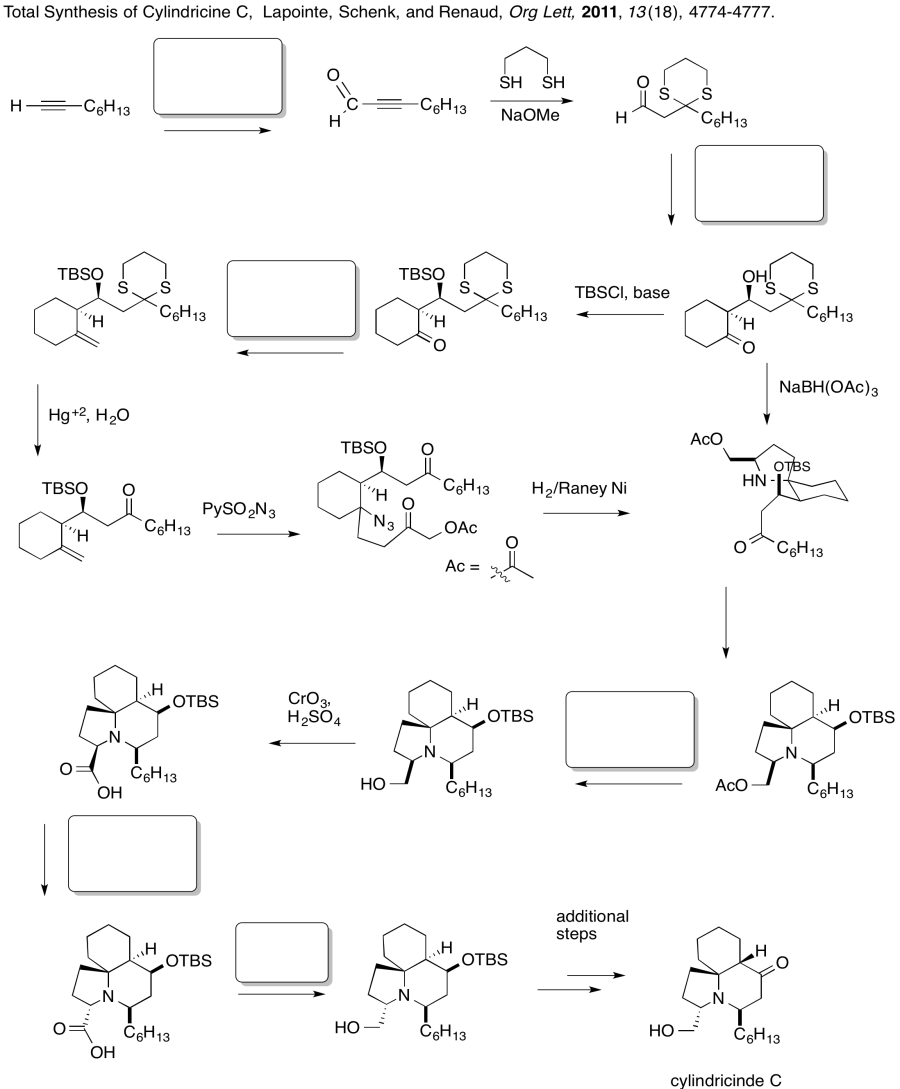
Problem CX12.6.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl , nucleophilic substitution at carboxyl and conjugate addition .
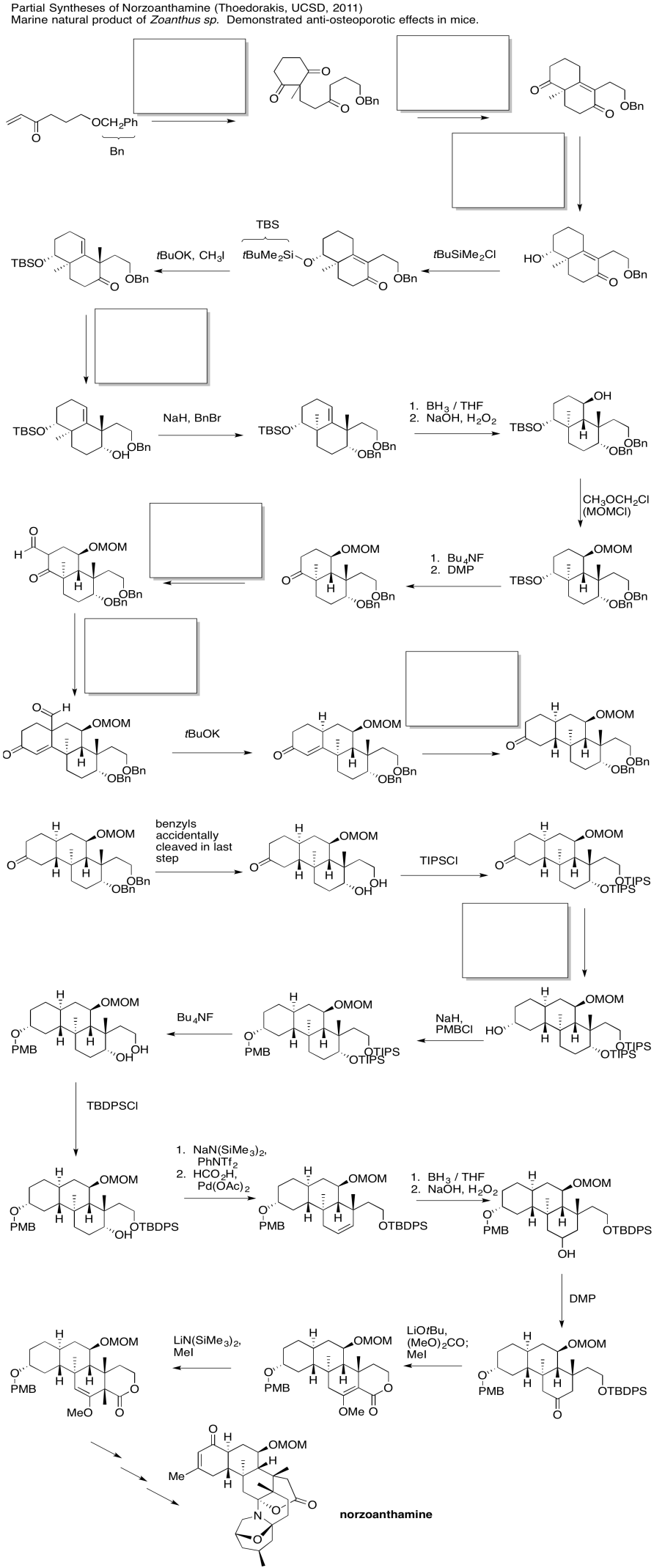
Problem CX12.7.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl, conjugate addition and nucleophilic substitution at carboxyl.
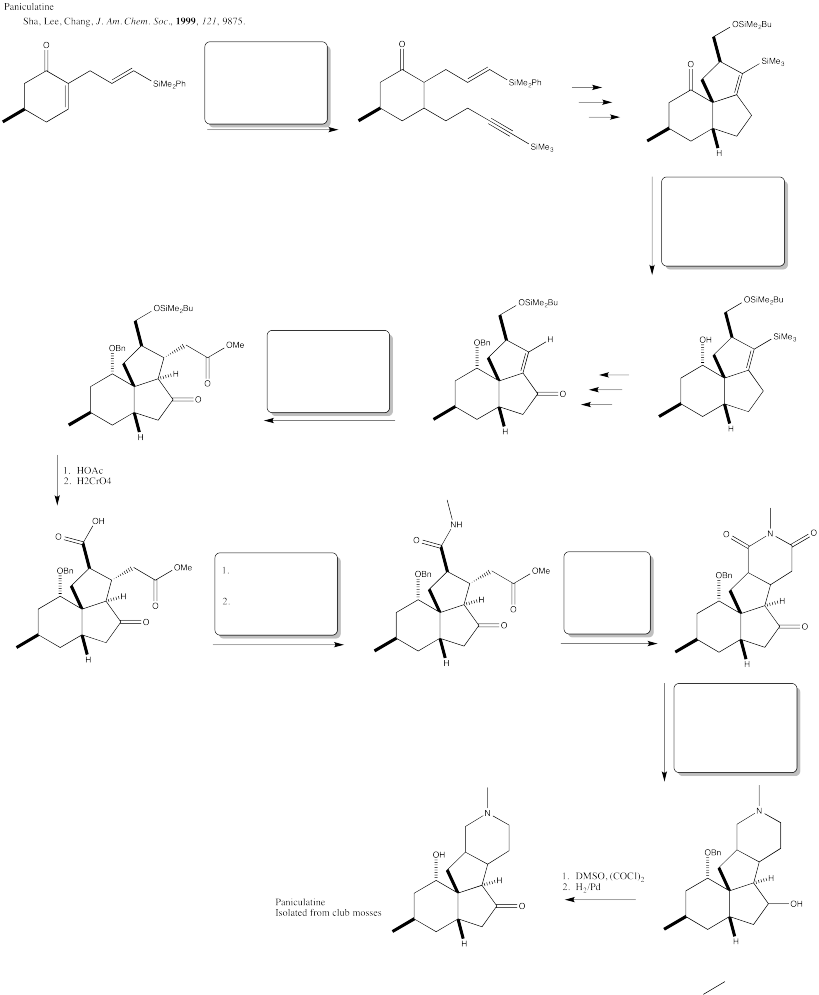
Problem CX12.8.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl, nucleophilic substitution at carboxyl and neutral nucleophilic addition to carbonyl.
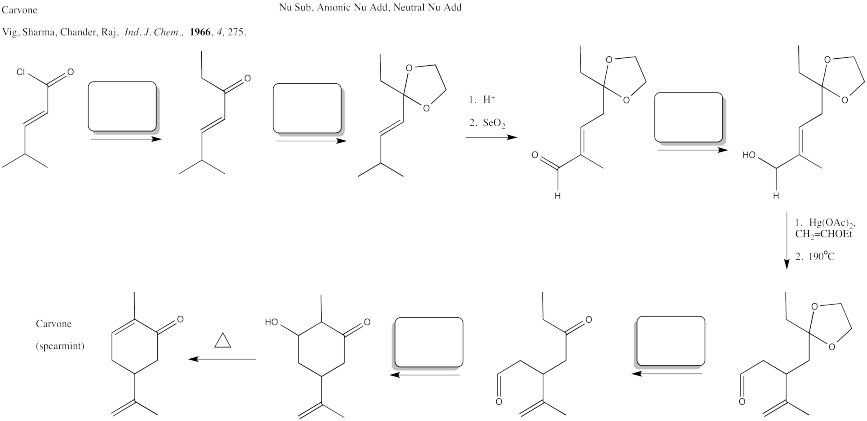
Problem CX12.9.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl/em>, conjugate addition, nucleophilic substitution at carboxyl and transition metal-catalysed coupling.
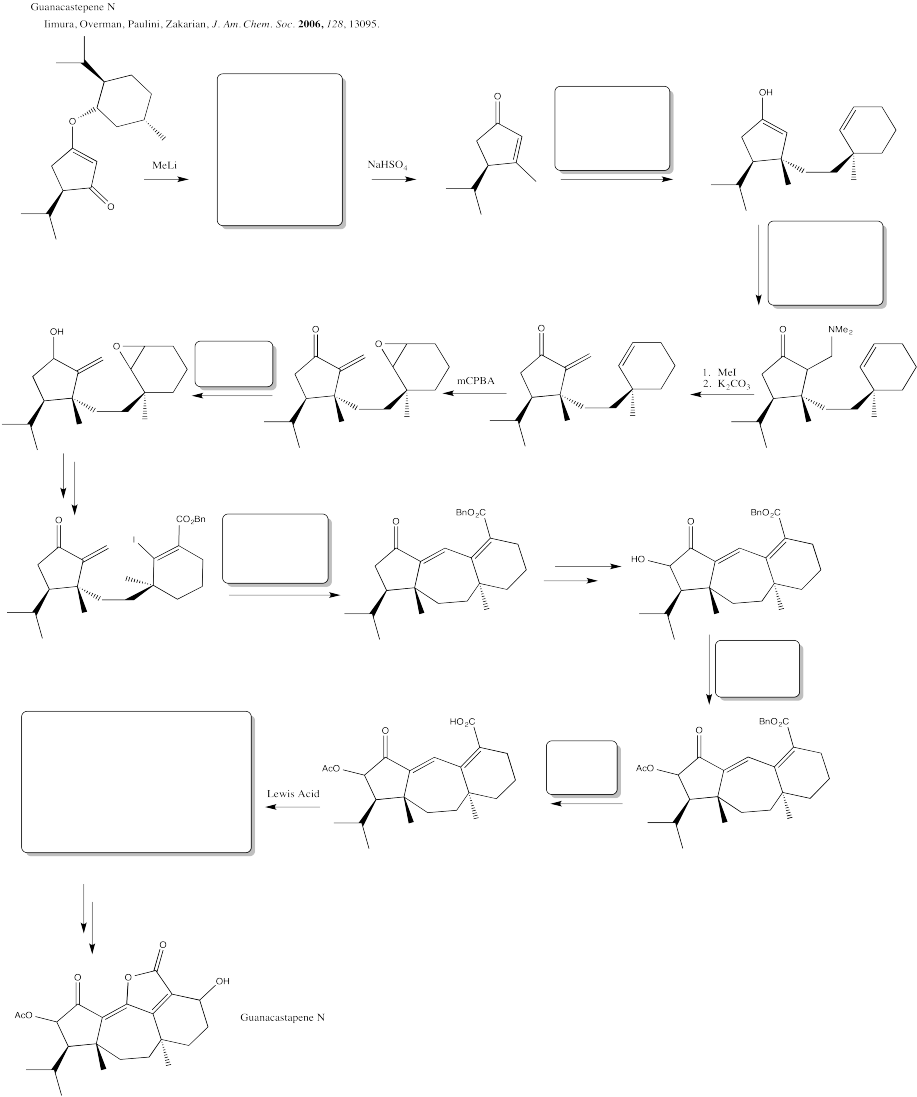
Problem CX12.10.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl, conjugate addition, nucleophilic substitution at carboxyl and transition metal-catalysed coupling.
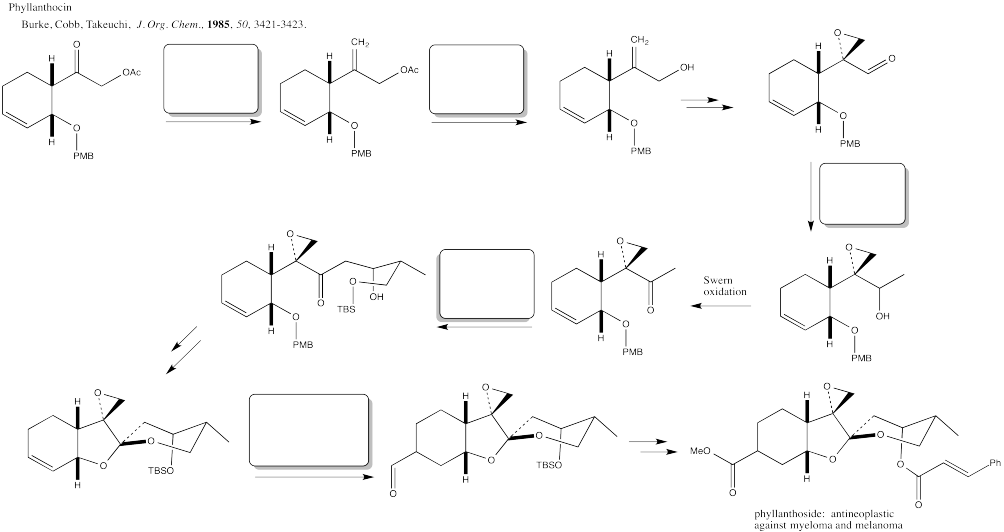
Problem CX12.11.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl, conjugate addition, nucleophilic substitution at carboxyl, transition metal-catalysed coupling and neutral nucleophilic addition to carbonyl.
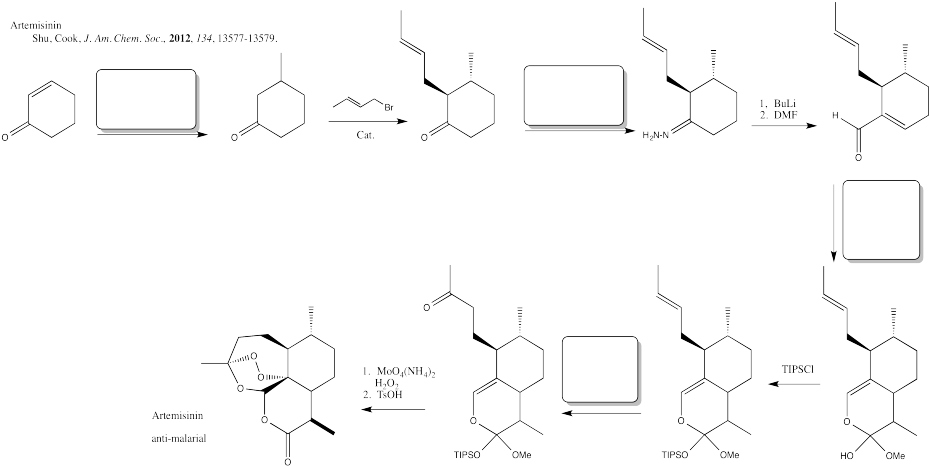
Problem CX12.12.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl, conjugate addition, nucleophilic substitution at carboxyl, transition metal-catalysed coupling and neutral nucleophilic addition to carbonyl.
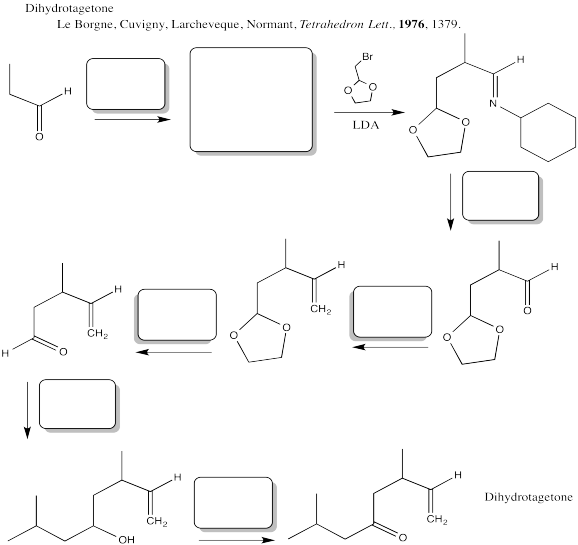
Problem CX12.13.
Fill in the blanks in the following synthesis involving anionic nucleophilic addition to carbonyl, conjugate addition, nucleophilic substitution at carboxyl, transition metal-catalysed coupling and neutral nucleophilic addition to carbonyl.
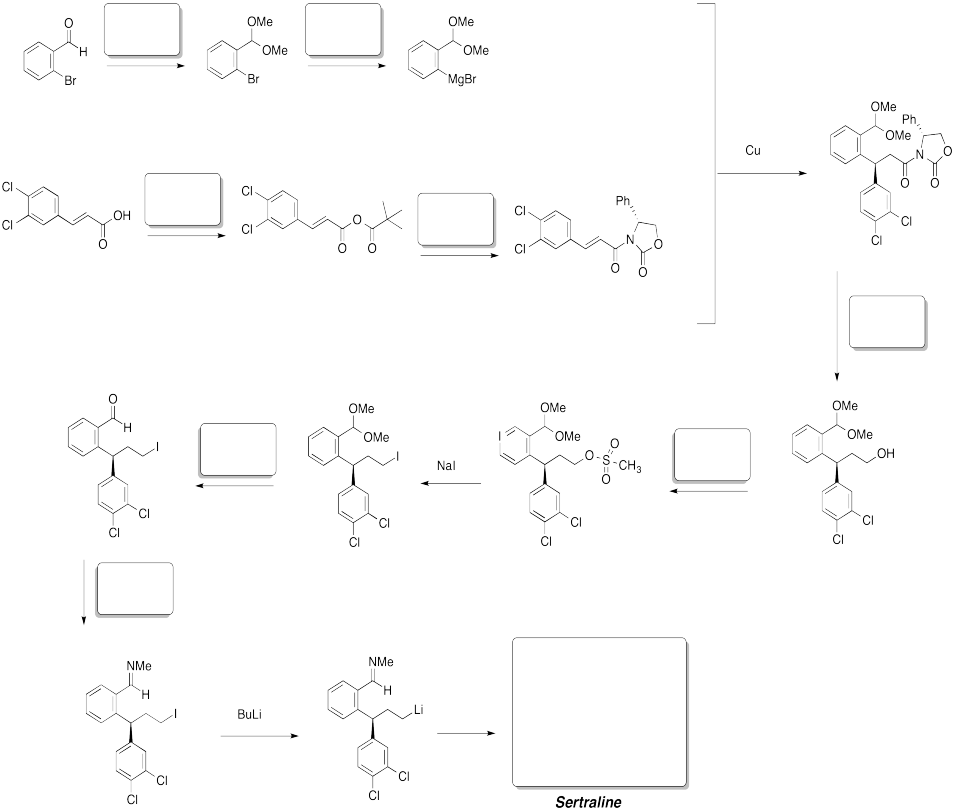
Problem CX12.14.
Fill in the blanks. Requires knowledge of formation of esters and amides as well as Wittig or Horner-Wadworth-Emmons reactions.
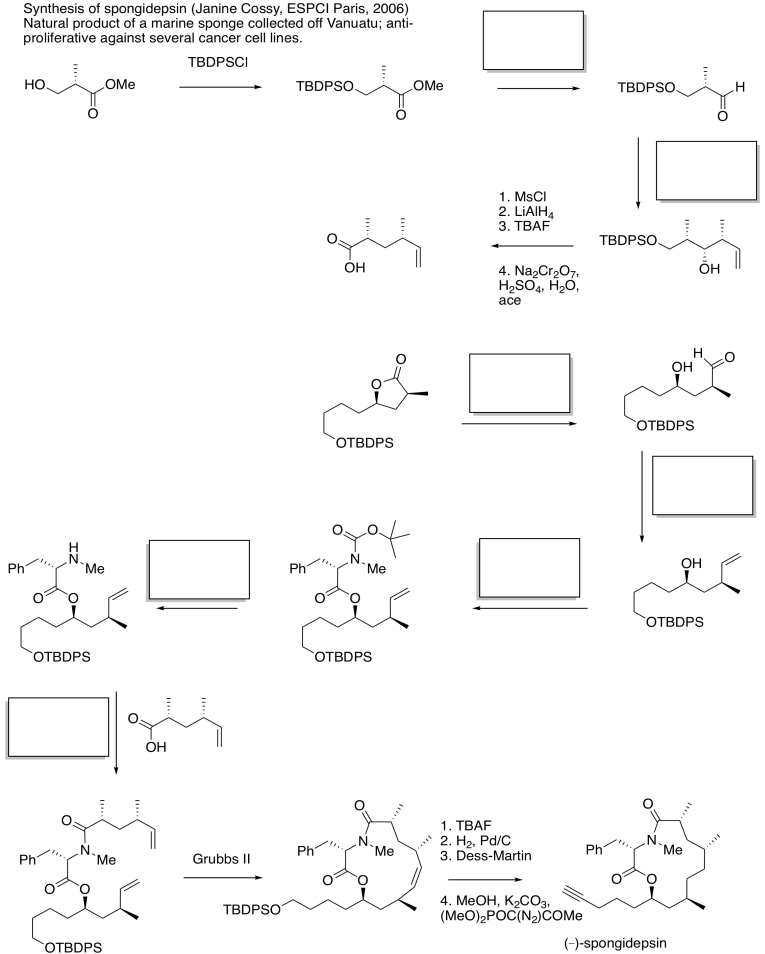
Problem CX12.15.
In each case, analyse whether the starting material is a nucleophile or electrophile. Fill in the product in part (c). This question requires knowledge of carbonyl conjugate addition.
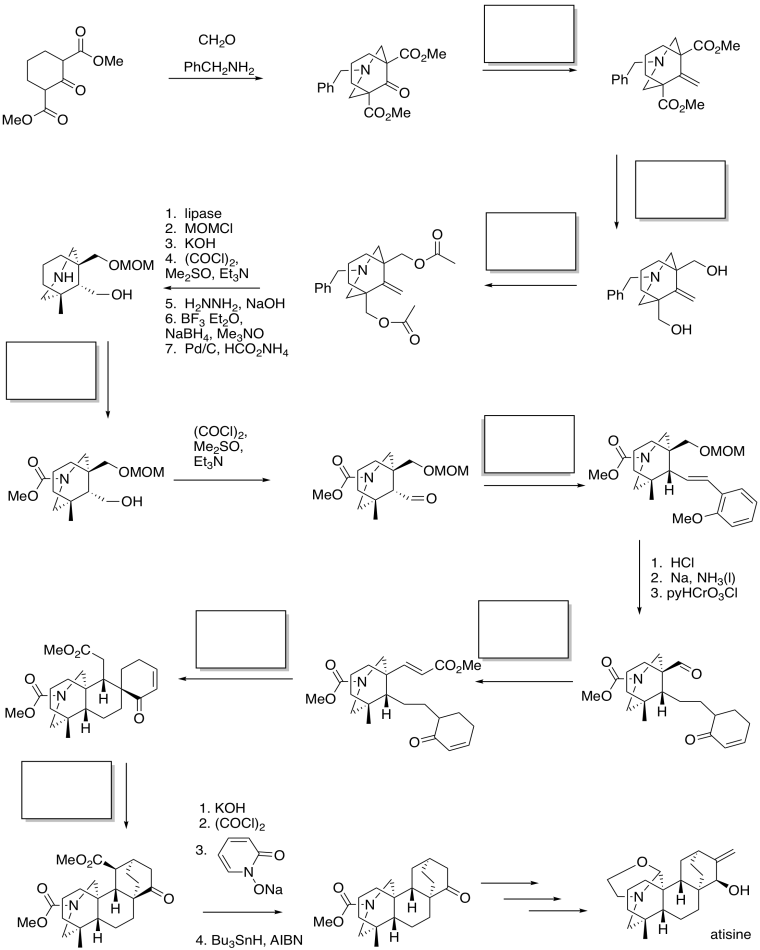
These strategies are used in Fukumoto's synthesis of atisine (below).
Problem CX12.16.
Fill in the blanks in the following synthesis of atisine (Keiichiro Fukumoto, Tohoku University). Atisine is a natural product of Aconitum sp., the family of poisonous plants that includes wolfsbane.
Requires knowledge of conjugate addition / Michael reaction, ester reductions, formation of esters and amides, and Wittig or Horner-Wadworth-Emmons reactions.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry