Reactivity in Chemistry
Substitution at Carboxyloids
CX9. Peptide and Protein Synthesis
Peptides and proteins are very important in biology. As a result, synthesis of these molecules has become very important, allowing for the laboratory study of model compounds that can give us insight into how proteins work, as well as pharmaceutically important compounds.
Insulin and glucagon are two important peptides (or small proteins) that regulate blood sugar. Insulin signals that blood sugar levels are high and that the body should begin storing this excess sugar. Glucagon signals that blood sugar levels are low and so the body may need to access its long-term energy stores. Both compounds are important in medicine.
Glucagon has a relatively simple structure, for a protein. It is a string of just 29 amino acids, connected together in the following order:
His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr.
The structure is composed of readily-available starting materials. The amino acids are simple units that connect together to form a chain. Conceptually, it seems easy to put two amino acids together to make a side chain. When two amino acids come together, they lose a molecule of water together, and the remaining pieces are able to bond to each other to make the dipeptide. It ought to be just as easy to add a third, and so on.

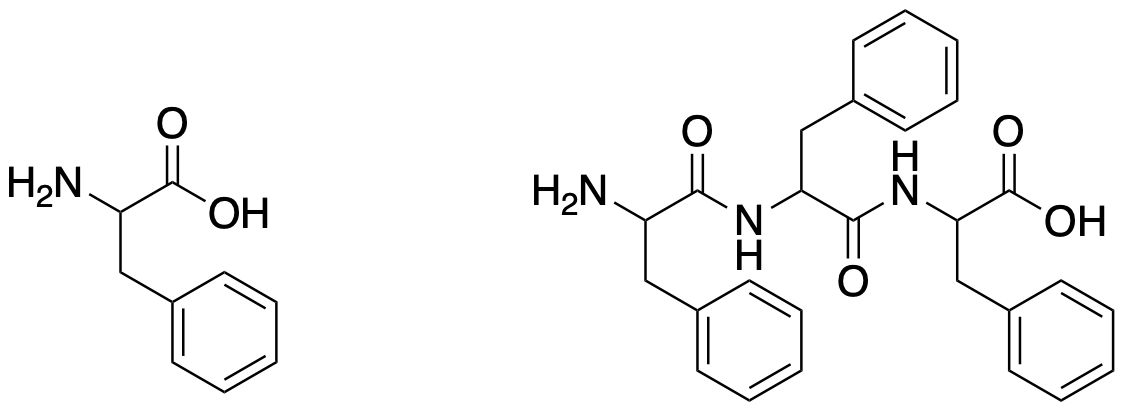
Figure CX9.1. An amino acid and a tripeptide made from it.
Structurally, amide or peptide bonds are very stable and resistant to carboxyl substitution. That stability makes optimal structures from which to construct proteins. Despite being composed of very long chains of linked amino acids, proteins actually have some limits on their conformational flexibility (their "floppiness"). That allows proteins to more reliably hold a particular shape. The shape of proteins is crucial to their function as enzymes and other
Both the stability and the structural rigidity of peptides arises from the nature of the peptide bond. The pi donation that hinders nucleophiles from substituting at the carbonyl is pronounced enough that it can be considered to form an additional bond. Thus, peptides behave as though they contain C=N bonds rather than C-N bonds. X-ray structure determinations show that the peptide nitrogens in proteins are trigonal planar, not pyramidal. In addition, many peptides exhibit cis-trans isomerism. For every peptide bond, two different isomers can occur, depending on whether a substituent attached to nitrogen is on the same side of the C=N bond as the carbonyl oxygen or the opposite side.
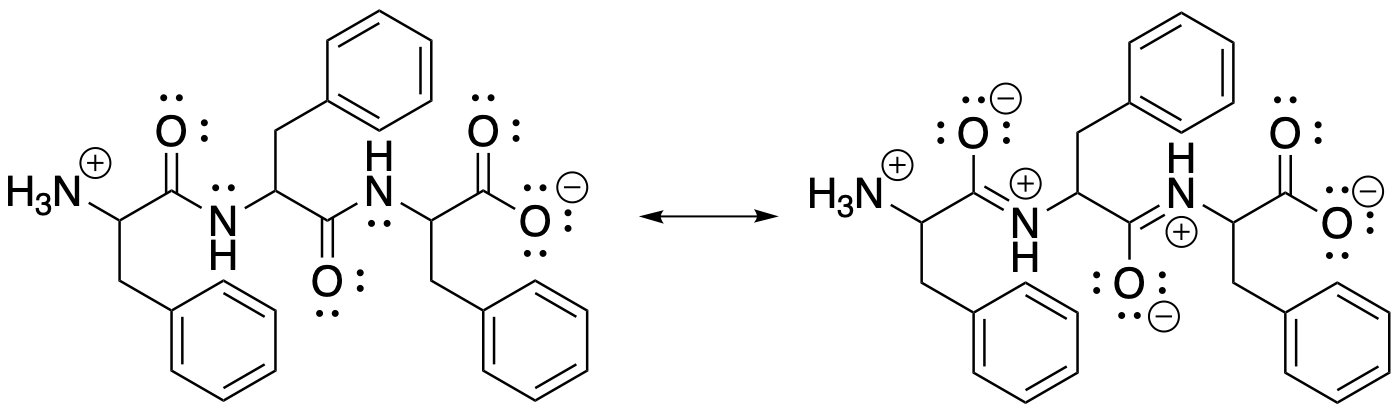
Figure CX9.2. Resonance in the backbone of a tripeptide.
The great stability of these structures does not mean they are easy to make. Part of the difficulty stems from the fact that amino acids are difunctional. In order to form long chain structures, amino acids must be able to react twice: once with an amine, to grow in one direction, and once with a carboxylic acid to grow in the other direction. In other words, an amino acid contains both a nucleophile and an electrophile.
Suppose we were to try to make the dipeptide, ala-phe. This peptide contains an alanine connected to a phenylalanine through a peptide bond. The peptide bond is formed between the carboxylic acid of alanine and the amine of phenylalanine.
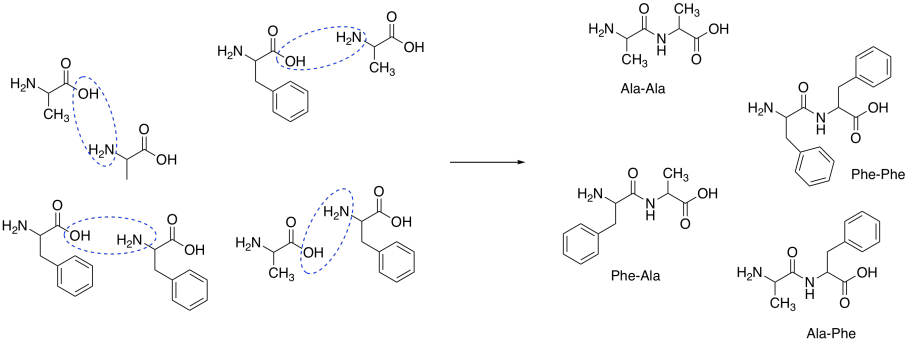
Figure CX9.3. Dipeptides made from a mixture of alanine and phenylalanine.
Assuming the amino acids do react together to form the peptide, combining these two reactants would likely produce a mixture of four dipeptides:
Ala-Phe Ala-Ala Phe-Phe Phe-Ala
In other words, peptide formation from amino acids is non-selective.
Problem CX9.1.
Draw structures for the four peptides formed by combining glycine and leucine.
Problem CX9.2.
What tripeptides would be produced by mixing Ala, Gly and Val?
Problem CX9.3.
Simply combining these peptides might not result in any peptide formation at all. Why not?
An additional complication in peptide synthesis is that amines and carboxylic acids do not really exist together. Instead, a proton is transferred from the carboxylic acid to the amine, forming a salt. The carboxylate is no longer very electrophilic, due to its negative charge. Because of its positive charge, the ammonium ion is no longer very nucleophilic.
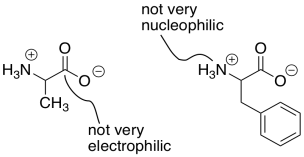
Figure CX9.4. The carboxyl group in an amino acid is deactivated as an electrophile.
As a result, there are actually two distinct problems in peptide synthesis. There is a selectivity problem, because each amino acid has a nucleophilic part and an electrophilic part. There is no way to ask one compound to react only using its electrophile and another compound to react only using its nucleophile. There is also a reactivity problem: the carboxyl group in this case is a terrible electrophile, and the amine is a terrible nucleophile.
In laboratory syntheses, a number of techniques have been used to make peptide synthesis selective. Most frequently, protecting groups are used. A protecting group "masks" one of the two functional groups on an amino acid, but leaves the other one open. If one amino acid has its amine protected, it can only react via its carboxylic acid. If the other amino acid has its carboxylic acid protected, it can only react via its amino group. Only one combination will result.
The key to protecting groups is that the reaction used to mask one of the functional groups must be reversible. You must be able to take the protecting group back off when it is no longer needed.
Carboxylic acids are normally protected as esters. Esters can be removed via acid- or base-catalyzed hydrolysis (as can amides, but esters are more reactive, being farther up the ski hill).
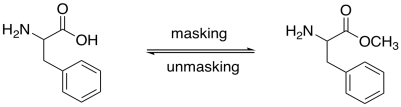
Figure CX9.5. Protection of the carboxylic acid as a methyl ester.
Amines are normally protected as amides. However, we need to be able to remove specific amides her: the ones that mask the amines, not the ones that we have formed to link two amino acids together. As a result, in peptide synthesis, amines are usually protected as carbamates. Carbamates can be cleaved more easily than amides.
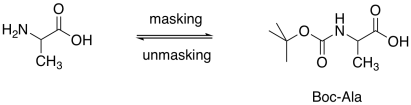
Figure CX9.6. Protection of the amine as a carbamate.
Problem CX9.4.
Propose a reason for the relatively higher reactivity of carbamates compared to amides.
Problem CX9.5.
Fill in the blanks in the following peptide synthesis.
To get around the problem of low electrophilicity of the carboxylic acid, a number of coupling agents have been developed. A coupling agent can temporarily convert the carboxylate anion into a more reactive electrophile. To do so, it exploits the nucleophilicity of the carboxylate anion. After donating to the coupling agent, the carbonyl compound becomes more electrophilic.
Thionyl chloride (SOCl2) can accomplish this goal, of course. It converts relatively non-electrophilic carboxylic acids into much more electrophilic acid chlorides. Thionyl chloride can be a little harsh, however, so chemists have sought to develop milder conditions that can knit two amino acids together without the necessity of forming a reactive acid chloride.
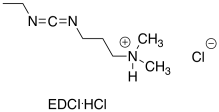
Some of the most commonly-used coupling agents for peptide synthesis are carbodiimides. These compounds contain an electrophilic N=C=N unit to act as an initial electrophile. Diisopropylcarbodiimide (DIC) and dicyclohexylacarbodiimide (DCC) were some of the earliest and simplest examples of these compounds developed for peptide synthesis.
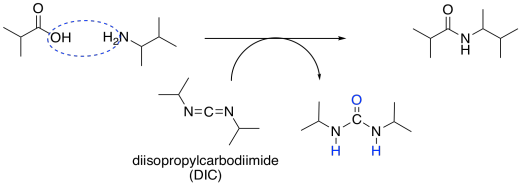
Figure CX9.7. Carbodiimide coupling to make an amide bond.
Once a carboxylate has donated to the electrophilic carbon of the carbodiimide, a better leaving group is formed. The adjacent nitrogen atoms act as basic sites, picking up a proton from the carboxylic acid on one amino acid and from the ammonium ion intermediate formed by the other amino acid.
Problem CX9.6.
Propose an advantage of EDCI as a coupling agent for amino acids, compared to DCC.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry