Reactivity in Chemistry
Substitution at Carboxyloids
CX6. Semi-Anionic Nucleophiles
Carboxyloids can be interconverted through addition of typical heteroatomic nucleophiles: amines, alcohols, thiols and water. In addition, other nucleophiles can displace the leaving group from a carboxyloid, provided the nucleophile is reactive enough.
Problem CX6.1.
Could a halide, such as bromide or chloride, replace a carboxyloid leaving group easily? Explain.
Carbon and hydrogen anions (or "semianions") are very good nucleophiles. Earlier, we saw how they can react with simple carbonyls. Although the lone pair on a carbon or a hydrogen is often masked in a covalent bond with a moderately electropositive metal such as aluminum or magnesium, that bonding pair of electrons is still nucleophilic enough to donate to a good electrophile. These nucleophiles can often react with carboxylic acid derivatives.

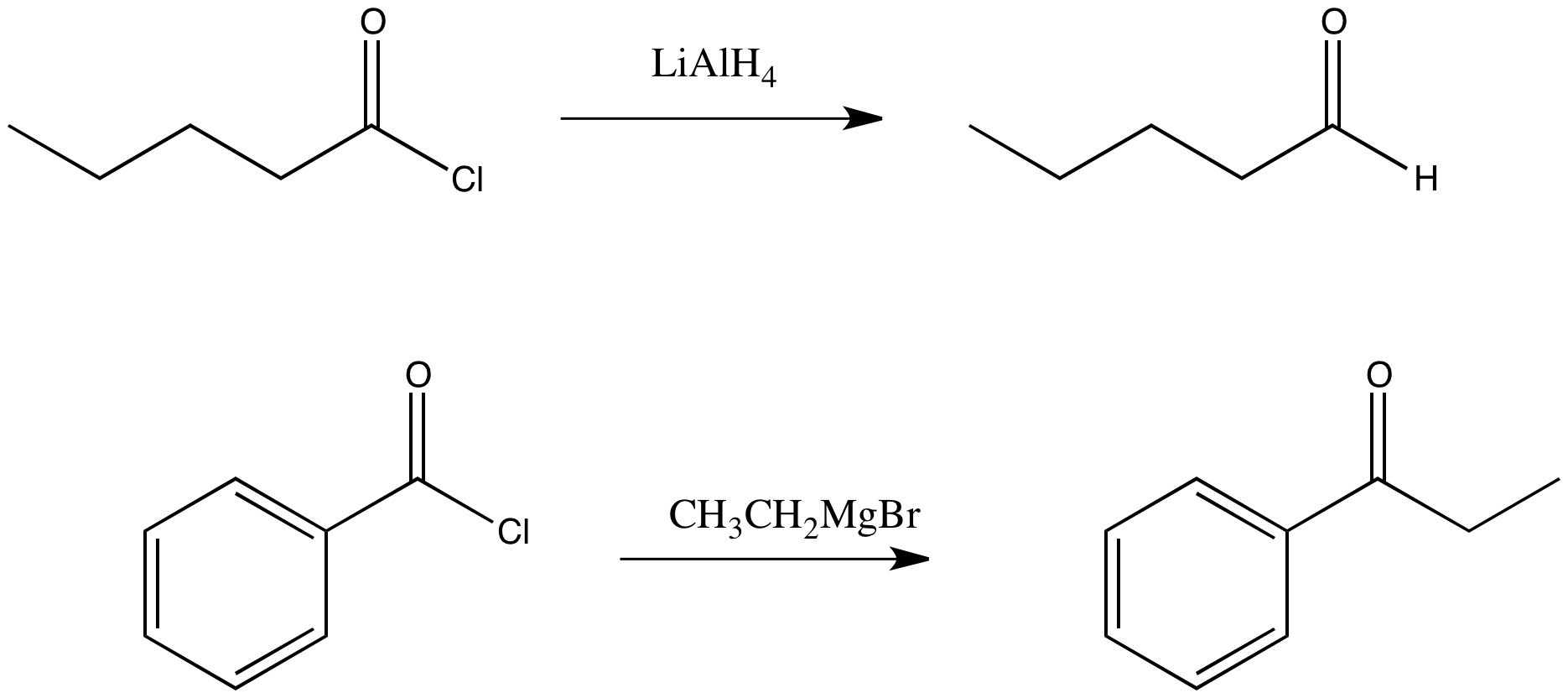
Figure CX6.1.The (seemingly) obvious substitution product of an acid chloride with a complex hydride and a Grignard reagent.
Problem CX6.2.
Draw a mechanism for the replacement of the chloride in propanoyl chloride with a hydride from lithium aluminum hydride.
Problem CX6.3.
Draw a mechanism for the replacement of the chloride in propanoyl chloride with a methyl from methylmagnesium chloride.
However, we are going to see that semianionic nucleophiles do not really follow this pattern of replacing the leaving group with the nucleophile. Although that does happen, the product of that reaction is an aldehyde or ketone. We have already seen that aldehydes and ketones react with semianionic nucleophiles, undergoing nucleophilic addition reactions to make alcohols. If a nucleophilic substitution of a carboxyloid leads to formation of an aldehyde, then that aldehyde will keep reacting under these conditions to form alcohols.
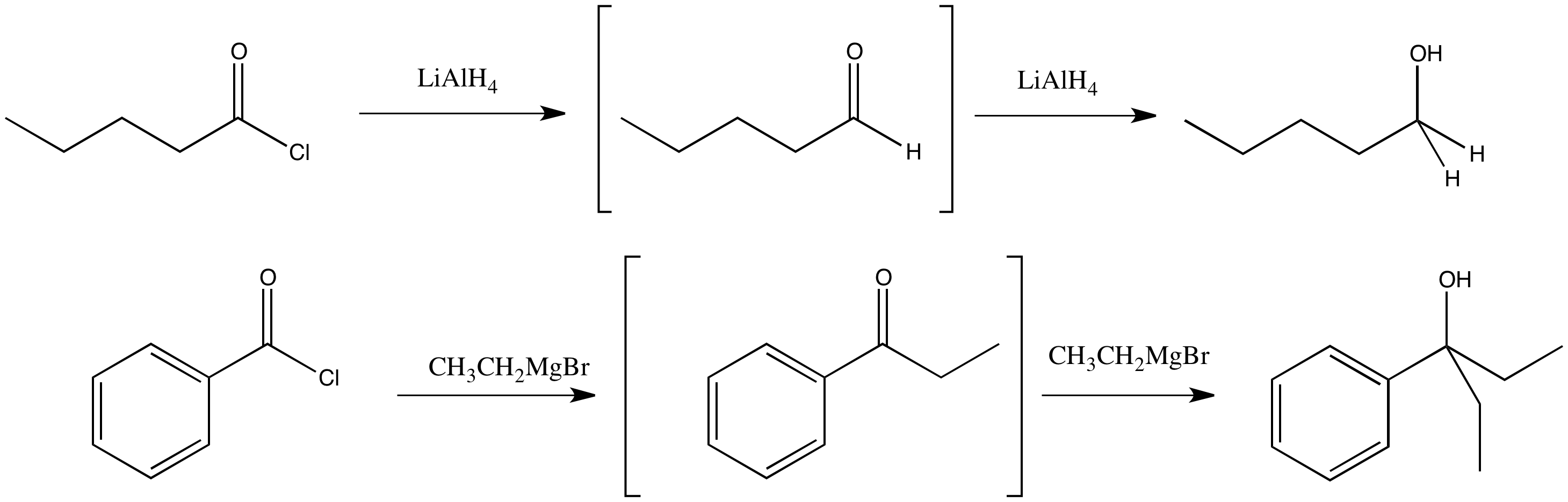
Figure CX6.2.The real substitution product of an acid chloride with a complex hydride and a Grignard reagent.
Not all hydride reagents are the same, however. Some are more reactive than others. The most common examples of complex hydride reagents are sodium borohydride and lithium aluminum hydride. Both are quite reactive species that are sensitive to traces of moisture, even in the surrounding air. The partially anionic, nucleophilic, basic hydride reacts with the partially positive and very mildly acidic hydrogen of a water molecule, producing H2 gas. Lithium aluminum hydride is much more reactive with water than sodium borohydride. The main reason for this increased reactivity is the increased electronegativity difference between aluminum and hydrogen compared to boron and hydrogen.
Not only is lithium aluminum hydride more reactive with water than sodium borohydride, but it is also more reactive with carbonyl electrophiles. Although both reagents react in an identical way with aldehydes and ketones, sodium borohydride is only sufficiently reactive to reduce the most reactive carboxyloids. It can reduce acid chlorides, anhydrides, and thioesters to aldehydes and then to alcohols, but it does not react at all with esters, carboxylic acids, amides, or carboxylate anions. In contrast, lithium aluminum hydride can reduce all of these carboxyloids.
Figure CX6.3. LAH can reduce esters, but NaBH4 cannot.
Sodium borohydride can reduce things like acid chlorides, aldehydes and ketones, but not things like esters or amides. It can be difficult to compare compounds that undergo different classes of reactions, but that means we could think of aldehydes and ketones as being better electrophiles than amides and esters, but not as electrophilic as acid chlorides.
Figure CX6.4. A modified potential energy surface that includes aldehydes and ketones.
Although lithium aluminum hydride reacts with amides, it does not result in formation of an alcohol. It is the only carboxyloid that fails to follow the usual patter of alcohol formation when treated with LAH. Instead, reduction of an amide with LAH leads to formation of an amine. In this case, the oxygen of the carbonyl eventually becomes the leaving group, rather than the amide nitrogen. This outcome has a parallel in the formation of imines and enamines in the addition of amines to aldehydes and ketones. In those cases, the carbonyl oxygen is also replaced. Like those cases, the loss of the carbonyl oxygen is related to the strong π-donating ability of the nitrogen. Another similarity is that the oxygen is lost after binding to an activating group. In the addition of neutral amines to aldehydes and ketones, th activating group is a proton that makes the oxygen a better leaving group. In the case of LAH, this role is filled by the aluminum, which is strongly Lewis acidic.
Figure CX6.5. The LAH reduction of an amide to an amine.
Addition of a Grignard reagent (alkylmagnesium halide) to a carboxyloid results in the formation of a ketone. Addition of a complex hydride reagent (such as lithium aluminum hydride) to a carboxyloid results in the formation of an aldehyde. We have already seen that these reagents can add to aldehydes and ketones to afford alcohols.
So, what happens if a Grignard reagent is added to a carboxyloid such as an acid chloride, an acid anhydride or an ester? The carboxyloid would be converted to a ketone. If there are still more Grignard molecules around, they would probably convert the ketone into an alkoxide ion (and ultimately an alcohol via protonation). The thing is, it is very likely that there will be more Grignard molecules around. A reaction tends to involve millions of reactant molecules, so by the time the first thousand or so molecules of carboxyloid have been converted to ketone, hundreds of those ketone molecules have already been converted to alkoxide.
Figure CX6.6. The addition of a Grignard reagent to an ester.
In most cases, alkyl reagents and hydride reagents will add twice to carboxyloids. They will convert the carboxyloid into an aldehyde or ketone. Because aldehydes and ketones also react with hydride and alkyl nucleophiles, they will react a second time.
However, just as sodium borohydride does not react with all carboxyloids, neither does an alkylmagnesium reagent. Grignard reagents do not react with the least reactive ones: carboxylic acids, carboxylate anions, and most amides. (There is an exception to the rule called a Weinreb amide, which is more electrophilic than other amides.)
In fact, there is a related alkylating reagent that is even more selective than a Grignard reagent. Dialkylcopper reagents such as Li[(CH3)2Cu] react easily with acid chlorides. They have also been reported to react with thioesters. In both cases, the result is a ketone. That is, these reagents only add once. That's consistent with the fact that they do not react with aldehydes and ketones, as alkyllithium and alkylmagnesium reagents do. They do not with the other carboxyloids such as esters, amides, and carboxylic acids.
Figure CX6.7. The addition of an organocuprate, or lithium dialkylcuprate, to an acid chloride.
Dialkylcopper reagents add only once to acid chlorides.
These organocopper reagents are complementary to organolithium and organomagnesium compounds in several ways. Organomagnesium and organolithium compounds react with aldehydes and ketones; organocopper compounds do not. Organocopper compounds can deliver alkyl nucleophiles in apparent SN2 reactions; organolithium and organomagnesium compounds cannot. When presented with α,β-unsaturated ketones (enones), organolithium and organomagnesium compounds react preferentially at the 1,2-position, the carbonyl itself, whereas organocopper compounds react only at the 1,4-position or conjugate position.
Figure CX6.8. The addition of a dialkylcuprate to an enone.
The mechanism of reaction with organocopper reagents is probably a little different than the mechanism of reaction of organolithium and organomagnesium reagents, contributing to the difference in selectivity. The probable pathways include oxidative addition / reductive elimination cycles or single electron transfer chemistry, but the exact mechanism is not something for you to worry about at this point.
Table CX6.1. A summary of reactions of alkyl reagents with carbonyl compounds.
| Electrophile | Organomagnesium or Organolithium | Organocopper |
|---|---|---|
| Aldehyde / Ketone | gives alcohol | no addition |
| Acid Chloride | gives alcohol | gives ketone |
| Conjugated Enone | adds 1,2- | adds 1,4- |
| Alkyl Halide | no substitution | substitution |
Acid chlorides are the most reactive carboxyloids. They react selectively with organocopper reagents when other derivatives do not. That raises a possibility: are there hydride reagents that are reactive enough to react only with acid chlorides, but not reactive enough to react with aldehydes and ketones? The answer is, not really. However, researchers have intentionally developed reagents that react very slowly with aldehydes and ketones compared to acid chlorides, with the intention of catching the reaction at a point when conversion to the alcohol has not happened very much.
How would they do that? They can use lithium tris(tert-butoxy)aluminum hydride, Li[(t-BuO)3AlH]. Remember, that means Li[((CH3))3CO)3AlH].
Figure CX6.9. Li(tBuO)3AlH, a selective hydride reducing agent.
Why is this reagent very slow to react with aldehydes and ketones? For one thing, it has three oxygens attached to the aluminum, whereas LiAlH4 has only hydrogens attached to the aluminum. The electronegative oxygens draw electron density towards themselves and away from the lone hydride on the aluminum. That makes the hydride less negative and less nucleophilic. The reaction slows down.
Figure CX6.10. The reaction of Li(tBuO)3AlH with an acid chloride.
The same thing could also be done with Li[(CH3O)3AlH], but Li[(t-BuO)3AlH] works better. That's because the tert-butyl groups are much bulkier than methyl groups. They make the nucleophile much more crowded as it approaches the electrophile, slowing the reaction down further. A third, very important factor is that this reaction must be run at very low temperatures to slow it down even further, such as -78°C, or else the reagent will continue to react with the aldehyde, anyway.
Figure CX6.11. Some mechanistic details in the Li(tBuO)3AlH reduction.
It may be more surprising to know that there is a reagent developed for the purpose of reacting with esters and stopping at the aldehyde. The reason this is surprising is because the ester is one of the least reactive carboxyloids. The reagent used is diisobutylaluminum hydride, DIBAL, ((CH3)2CHCH2)2AlH.
Figure CX6.12. iBu2AlH, a selective hydride reducing agent.
This reagent, if used very carefully, can reduce an ester to an aldehyde. The reaction has to be carefully controlled, keeping it at -78°C before warming it up to 25°C. Reportedly, the reaction is finicky and can be very difficult to reproduce.
Figure CX6.13. The reaction of iBu2AlH with an ester.
The approach in this case doesn't depend on stopping the reaction based on relative reactivity. It depends on a chelate effect. Remember, in coordination chemistry, chelation is when a ligand binds to a metal via two different donor atoms at the same time. Chelation makes coordination complexes hold together a little more firmly, like holding onto something with two hands instead of one hand can give you a better grip. An ester is a good candidate for a chelating ligand because it has two oxygen atoms with lone pairs. It can be a bidentate donor to aluminum. Aluminum is pretty oxophilic (oxygen-loving); it is always found as aluminum oxides in nature. An ester can bind tightly to an aluminum with its two oxygens, and that's the key to how this reagent works.
Figure CX6.14. Some mechanistic details in the iBu2AlH reduction.
In this case, when the hydride donates to the carbonyl, the leaving group doesn't leave, because it is held in place by the aluminum to which it is bound. This interaction is enhanced by the fact that the aluminum in DIBAL is only three-coordinate, not four-coordinate as in LiAlH4. The open coordination site allows the two oxygens to bind more easily to the aluminum and arrest the reaction at an intermediate stage. When water is added to wash the aluminum away, the chelating complex breaks down. With nothing left to held the alkoxy leaving group in place, it can leave. An aldehyde is formed, but at that point the hydride agent has been broken down by the water, so there is no opportunity for further reaction to an alcohol. Just as in the case of lithium tris(tert-butoxy)aluminum hydride and acid chlordies, this reaction has to be run at low temperature. Even so, both reactions are difficult to control, and they don't always stop when they are supposed to.
Problem CX6.4.
Grignard reagents will not effect leaving group replacement in carboxylic acids. Show why that particular reaction does not occur, with the help of a mechanism.
Problem CX6.5.
Grignard reagents generally do not react with either amides or carboxylate ions. Explain why.
Problem CX6.6.
Provide a mechanism for the reaction of propanoyl chloride with lithium dimethylcuprate, LiCuMe2. Will the reaction proceed to an alcohol, like a Grignard reagent would?
Problem CX6.7.
Complex hydride reagents can be very selective towards carboxyloids. For example, sodium borohydride is not powerful enough to react with esters.
a) Which of the carboxyloids can sodium borohydride react with? Explain.
b) What other carboxyloids can sodium borohydride NOT react with? Explain.
Problem CX6.8.
Lithium aluminum hydride can induce carboxylic substitution with carboxylate salts such as sodium butanoate.
a) What would be the ultimate product of this reaction? Explain.
b) What other carboxyloids can lithium aluminum hydride react with? Explain.
c) The aluminum assists in removing the oxide leaving group from this compound. Show how that happens with a mechanism.
Problem CX6.9.
a) Which is more reactive: lithium aluminum hydride or sodium borohydride?
b) Which is more selective: lithium aluminum hydride or sodium borohydride?
c) How can you explain the difference in reactivity between lithium aluminum hydride and sodium borohydride?
Problem CX6.10.
The reaction of lithium aluminum hydride with amides is unusual in that the final product of the reaction is generally an amine.
a) Why does this reaction seem to be different from other carboxyloid reactions?
b) Draw a mechanism for this reaction. (Hint: at some point, an oxygen atom donates a pair of electrons to aluminum.)
c) Propose a reason why this hydride reaction follows a different path than other reactions of hydrides with carboxyloids.
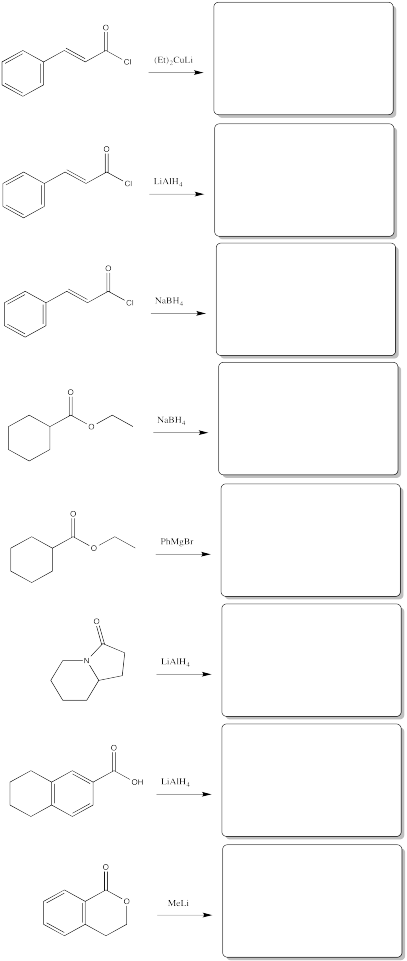
Problem CX6.11.
Fill in the products of the following reactions.
Answers to selected problems are found here.
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carboxyl Substitution Index
Back to Web Materials on Structure & Reactivity in Chemistry