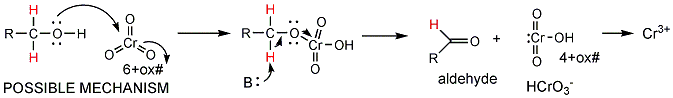CH 105 - Chemistry and
Society
Ethanol: Biochemistry
03/10/2007
Consider this a review of the biochemical
properties and activities of ethanol, with special emphasis to the biochemistry
topics we discussed in class.
Science,
Atoms, and Molecules
- Ethanol is a molecule (the smallest
particle of a compound that possesses the chemical properties of the
compound; a definite, distinct, electrically neutral group of bonded atoms)
that consists of carbon, oxygen and hydrogen atoms. These atoms are
nonmetals.
- Our experience from the alcohol industry
tells us that ethanol is soluble in water, since two distinct phases are not
seen when ethanol is mixed with water as is observed when oil and water are
mixed. A quick demonstration shows that it evaporates more readily
than water. A solution (homogeneous mixture) of water and ethanol can
be separated by distillation, in which the mixture is heated. The
ethanol evaporates more readily and can then be collected in the liquid form
by condensation on a cold surface (such as a glass tube with cold water
running through it).
Bonding
- It is held together by covalent bonds
involving the sharing of electrons between the bonded atoms. (insert
Lewis structure).
- In the Lewis structure, the carbon and
oxygen atoms are surrounded by eight electrons (an octet of electrons, or
four pairs), leading to the stability of these atoms in comparison to atomic
C and O which have 4 and 6 outer shell electrons respectively. (C is
in group 4 and oxygen in group 6 of the periodic table.) H has a duet
of electrons. Since these atoms are nonmetals, they achieved filled
outer shell through the sharing of electrons.
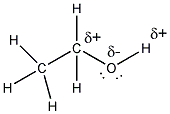
- There are two types of bonds in ethanol.
The C-H bonds are nonpolar covalent, since these atoms are of similar
electronegativity (the ability of an atom to attract electrons to
itself when it is bonded to another atom in a compound.) The C-O and
O-H bonds are polar covalent since O is significantly more electronegative
than either C or H. Hence there is a slight + charge (d+) on the C and
H attached to the O and a slight - charge (d-) on the O.
- The formal charge on each atom in ethanol
is zero. This is calculated by assigning electrons to each atom in
ethanol and comparing the number assigned each atom to the number found in
the outer shell of the lone atom unbonded. Lone pairs are assigned to
the atom they are placed around in the Lewis structure. Bonded
electrons are divided equally between the two atoms in the bond. If
atom in a molecule are assigned more electrons than the lone atoms has in
its outer shell, it would have negative charge equal to the excess number of
electrons. Positive charges develop when they are assigned fewer.
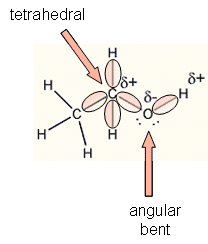
- With 8 electrons (4 pairs) surrounding the
C and O of ethanol, the geometry of the electrons clouds around the C and O
are tetrahedral, with bond angles of approximately 109o. In
this arrangement, the electrons clouds, which repel each other, are as far
apart as possible. Since 4 atom are attached to each carbon, the
geometry of the atoms around the C is also tetrahedral. However, only
two atoms (C and H) are attached to the O, so the geometry of the atoms
around the O is bent or angular. This geometry shows that their is a
separation of the center of slight positive and slight negative in the
molecule, which makes this molecule polar.
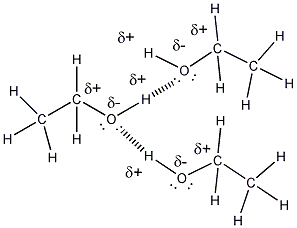
Intermolecular Forces and Solutions
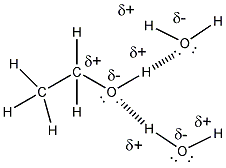
- Ethanol is a liquid at room temperature,
implying that ethanol molecules can attract other ethanol molecules with
strong enough intermolecular forces that the substance remains a liquid at
room temperature. Part of ethanol (CH3CH2- is nonpolar
and might be thought to make ethanol insoluble in water. However, the
C and H directly bonded to O are slightly charged, making this end of the
molecule polar. As mentioned above, the molecule, given its small
size, is polar. This property makes water soluble in water. Its
nonpolar part does also contribute to its solubility in nonpolar substances
such as gasoline (in gasohol). I'm not certain if additive are
required to increase it solubility in nonpolar gasoline.
- ethanol molecules can attract other
ethanol molecule and water through H bonds. The nonpolar ends of
ethanol would attract nonpolar molecules such as those found in gasoline
through induced dipole - induced dipole interactions (London forces).
Chemical reactions
- Ethanol can participate in redox reaction
with a variety of oxidizing agents. It can be burned (combustion
reaction) with O2 (g) in a redox reaction which produces CO2(g)
and H2O(g). In this case oxygen gas serves as the oxidizing
agent (causes ethanol to lose electrons). O2 (g) is then
reduced. This loss (oxidation) and gain (reduction) of electrons
can be mostly readily determined by calculating the oxidation number for
each atom in the molecules. Oxidation number represent another way to
count the electrons around atoms in molecules and ions (sort of another kind
of charge). The are calculated in the same way as formal charge
(described above) only bonded electrons (shared pairs) are given entirely to
the more electronegative atom (usually F, O, or N) in the bonded pair.
If two identical atoms are bonded together, the electrons in bond between
them are divided equally between the two identical atoms. Oxidation of
ethanol with O2 can be used to produce energy to drive vehicles
or to power our muscles when we drink it.
- Ethanol can participate in acid/base
reactions under some conditions. The H attached to O in ethanol is
slightly positive and could be attacked by a strong base which abstracts the
proton from the OH group on ethanol, leaving a negative on the
electronegative O. Since the O is electronegative, and tends to pull
electrons toward it, this negative on O makes this product somewhat stable.
However, compare the acidity of ethanol to another two carbon molecule,
acetic acid. It can give up a proton on the OH to form acetate.
In this case, the negative on the O can be "diluted" through a process
called resonance so that it is distributed over both Os on the acetate ion.
This results in a lower negative charge density in the acetate, making it
more stable than the negative ion resulting for ethanol acting as an acid.
Hence acetic acid (which is a weak acid, and weak electrolyte) is a much
stronger acid than ethanol and we don't consider ethanol as an acid.
Cells and Signal Transduction
- Ethanol potentiates Cl- in flow through
GABA A channels, making the neuron more refractory to activation (i.e the
neuron is hyperpolarized by making the transmembrane potential more
negative). Hence it inhibits neuron firing.
- Ethanol inhibits one type of glutamate
excitatory neurotransmitter channels, the N-methyl-D-aspartate channel.
It binds to a different site on the channel than does glutamate. (JBC, 49,
pp 48815, 2003)
- Ethanol appears to activate
cAMP pathways in neurons. as well.
- In addition, it activates K+ channels,
promoting efflux of K+ ions and making the transmembrane potential more
negative, inhibiting neural firing. (Cell, 115, pg 655, 2003)
Organic Chemistry
- Ethanol is an example of an organic
molecule containing the alcohol functional group, ROH. Other
common functional groups include aldehydes, ketones, amines, carboxylic
acids, and carboxylic acid derivatives including anhydrides, esters, and
amides.
- Direct oxidation (burning) of ethanol by
oxygen to produce carbon dioxide and water is a fairly uncontrolled reaction
that produces much energy. The oxidation process can be controlled
more readily and produced smaller increments of energy loss when other
oxidizing agents are used. In the lab, CrO3 can be used to
oxidize alcohols like ethanol acetaldehyde, which can be further oxidized to
acetic acid.
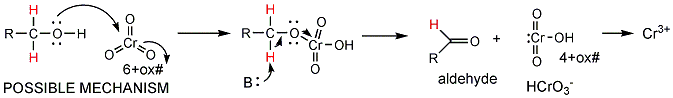
Biochemical Structures
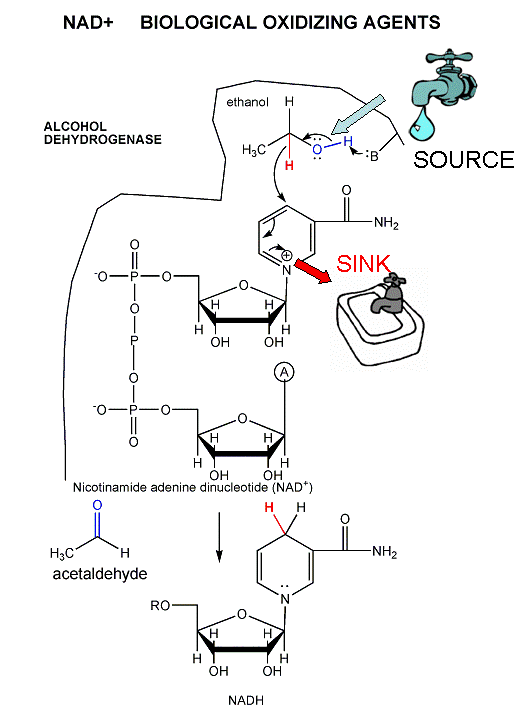
- Ethanol is metabolized in the body using a
different oxidizing agent, NAD+, to produce acetaldehyde.
This reaction is catalyzed by the enzyme
alcohol dehydrogenase
(ADH). There are 5 different versions of ADH in the body. Two of
these, which convert ethanol very quickly to acetaldehyde, are found often
in Asians. The quick generation of the toxic acetaldehyde produce
unpleasant symptoms which probably contribute to the decreased use of
ethanol in Asian cultures.
-
acetaldehyde is further
oxidized to acetic acid in a reaction catalyzed by another enzyme,
acetaldehyde dehydrogenase found in the liver. There are two different
forms of the enzyme, one found in the cytoplasm of the cell and the other in
the mitochondria. Half of all Asian people do not have a function form
of the liver enzyme, which leads to increased cellular levels of the toxic
acetaldehyde and a noticeable flushing in the skin. .
Central Dogma Biology
- Ethanol alters gene expression in target cells,
similar to other addicting drugs. We saw that such drugs affect the
reward centers of the brain, mediated by the ventral tegmental area (VTA)
and the nuclear accumbens (NA). VTA activation causes dopamine release
at the NA neuron. One effect of this is activation of cellular adenylate
cyclase which increases intracellular levels of the second messenger molecule cAMP.
Hence the dopamine receptor, of which there are at least five types, are not
neurotransmitter-gated ion channels. Rather on binding to dopamine and
subsequent shape changes, the receptor binds to and activates adenylate
cyclase, which makes cyclic AMP (cAMP). The interaction between the
receptor and adenylate cyclase is mediated by a G protein (so named since it
binds GTP). The cAMP second messenger activates Protein Kinase A which transfers a phosphate from ATP to many
proteins, including the cAMP Response Element Binding Protein (CREB), a
transcription factor. When phosphorylated this protein is activated
binds to the cAMP response element (CRE) upstream from certain genes, which
are then transcribed. One is dynorphin, a morphine-like molecule,
which acts to inhibit firing (and hence release of dopamine) from VTA cells
at the vTA-NA synapse. This reduction in dopamine elicits a response
(tolerance, craving, dependence) that causes increased drug use to get the
same psychological effects.
- Another protein transcription factor in
the NA neuron, delta-fosB, is activated by phosphorylation through the same
pathway. This protein is more stable and stays active in neurons weeks
after drug use has stopped. It can alter gene transcription in a long
term sense, and may account for sensitization responses for drug use, when
lower amounts of drugs and a variety of social and environmental cues can
lead to "falling off the wagon".
-
http://www.jneurosci.org/cgi/content/full/19/9/3277?ck=nck
Stoichiometry