Section 04A: MWF, 9:30-10:25 am. ASC 204, Crn# 12926
Section 05A: MWF, 10:40-11:35 am. ASC 104, Crn # 12927
Dr J's Contact Information
office: Ardolf Science Center 241
office hours: Tuesday 9:30-10:30 | Wednesday 3:30-4:30 | Thursday 9:30-10:30
telephone: 363-5354
email: hjakubowski@csbsju.edu (preferred method of communication)
How should you contact me
When you email me, include your section number (see below) in the subject line or otherwise I won't respond to your email:
125-04 if you are in my 9:30 AM section
125-05 if you are in my 10:40 AM section.
Last Update: 09/09/2014
Table of Contents
Resources
125-04A (9:30): CHEM125Tutor_F14_04A | CHEM 125-04A
125-05A (10:40): CHEM125Tutor_F14_05A | CHEM 125-05A
OFFICE HOURS
Darling models |
Student kit by Arbor Scientific
CHEM 125 is our single introductory course in chemistry taken by science majors and students in allied and prehealth programs. By itself it does not fufill the NS requirement of the common curriculum. Students must complete both CHEM 125 and CHEM 201 in order to earn the NS designation. It is not usually taken by nonscience majors seeking to fulfill the NS Common Curriculum requirements. Nonmajors usually take CHEM 105, Chemistry and Society, or a similar nonscience majors courses in other departments.
Tentative Daily Schedule (PDF)
This course will not be a repeat of high school chemistry.
This course will cover the structure and properties of chemical
compounds. We will look at
bonding and molecular properties, shapes of compounds, isomers and
stereochemistry, reactivity.
Atomic Structure and Periodic Trends
Metallic Structure and Properties
Ionic Structure and Properties
Molecular Structure:
Bonding and Geometry
Molecular Structure:
Properties
Molecular Structure:
Isomers
Biological Structures
Network Solids
Coordination Compounds
Molecular Bonding revisited:
Molecular Orbitals
Reactivity:
Acid-Base Chemistry
Quizzes/ Problem Solving Assessments (PSA)- 45%
Final Assessment - 15%, TBA
Sapling assignments - 20%
Graded homework/ individual and group - 10%
Prep and Participation: 10% (50 pt total with Moodle Tutor scaled to 25 pt, Socrative scale to 15 pt, and Group Dynamics/Participation to 10 pt)
TOTAL - 100%
In general, the following grading cutoffs will be used. Grade cutoffs won't be higher than shown.
Some of the grading derives from in-class activities. Because of this, ATTENDANCE IS MANDATORY! The group work will vary from informal, ungraded exercises to formal, graded assignments. Homework (in addition to Connect and Moodle assignments) may be assigned for chapters. I encourage you to work in groups. No late homework assignments will be accepted for grades. Quizzes/PSAs will usually be announced during in advance. Some grades will be group grades. The final assessment will emphasize the material presented after the last exam, but will also be cumulative.
I will not give makeup PSAs. If an extenuating circumstance arises (documented illness, family emergency, etc), I will allow you to use the score on the next PSA for the one you missed. Under specific situations, one Problem-Solving Assessment score may be dropped and replaced with the score on the next following cumulative PSA (which would probably include material from the missed subsequent PSA). This policy is in place for unforeseen incidences. Sometimes a student falls ill unexpectedly or a family crisis occurs or a Problem-Solving Assessment is lost. I will replace the percent score on your lowest PSA with the percent score Final Exam score if the final exam percent score is higher. This should give you incentive to perform better if you had a low PSA score.
GOALS AND STUDENT OUTCOMESThe general goals of the course can be broken into content and process goals, as described below.
Content Goals: Students should be able to:
explain how the distribution of electrons in atomic orbitals around atoms determine the physical and chemical properties of atoms;
explain how electrons redistribute around atoms in the process of bond and molecule formation;
draw representations (Lewis structures) of molecules that show the connectivity, bond types, and electronic and geometric arrangements of electrons and atoms within molecules;
use Lewis structures of molecules to predict and explain the physical properties (solubility, etc) of molecules;
explain and interpret bonding in molecules in terms of molecular orbitals;
recognize, explain, and predict how shapes of similar molecules that differ in connectivity of atoms or in in the spatial orientation of bonds influences their properties;
use Lewis structures of molecules to predict and explain the chemical properties (acid/base) of molecules.
These can be summarized in 4 general goals:
To gain an understanding of atomic structure and how the periodic table provides an organizing principle for the chemical and physical properties of the elements.
To develop an understanding of ionic, metallic and covalent bonding and how bonding affects properties.
To understand how
shape and charge distribution affects the properties of chemical
To learn how molecular structure predicts the acid-base properties of chemical species.
develop their abilities to transfer their newly acquired understandings of the structure and properties of simple molecules to more complex molecules and molecular systems;
develop critical thinking and problem solving skills
develop group interaction skills; (How important are these skills?)
explain how scientific understandings develop and how the scientific process differs from other ways of explaining the world. (A cool link on scientific literacy)

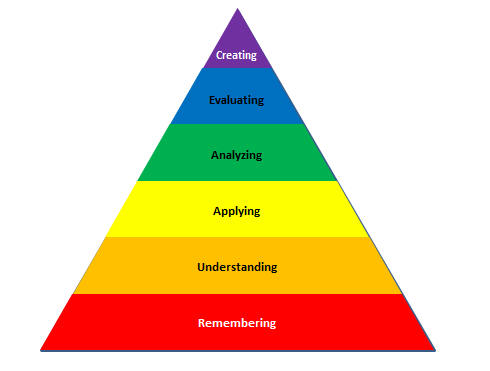
Remembering: can the student recall or remember the information? define, duplicate, list, memorize, recall, repeat, reproduce state
Understanding: can the student explain ideas or concepts? classify, describe, discuss, explain, identify, locate, recognize, report, select, translate, paraphrase
Applying: can the student use the information in a new way? choose, demonstrate, dramatize, employ, illustrate, interpret, operate, schedule, sketch, solve, use, write.
Analyzing: can the student distinguish between the different parts? appraise, compare, contrast, criticize, differentiate, discriminate, distinguish, examine, experiment, question, test.
Evaluating: can the student justify a stand or decision? appraise, argue, defend, judge, select, support, value, evaluate
Creating: can the student create new product or point of view? assemble, construct, create, design, develop, formulate, write.
Assessment in this class will include problems and question that require remembering, understanding, applying and analyzing.
How do we know if you have accomplished a goal that states that you will understand a specific area of chemistry? To access a goal, specific outcomes must be specified. These outcomes typical state that students will be able to do something, and are denoted by specific action verbs such as draw, predict, graph, describe, use, write, etc. Here is a more detailed document describing the CHEM 125 Learning Goals and Student Outcomes Used for all section of CHEM 125.
Recent developments in cognitive learning theory and
classroom research suggest that most students experience improved learning when
they are actively engaged and when they are given the opportunity to construct
their own knowledge. These results counter the widespread misapprehension that
effective teaching must be instructor-centered, involving the transfer of
content directly from the expert (professor) to the novice (student).
More "student-centered" approaches to learning are based on the premises that students will learn better when they are actively engaged in class and draw conclusions by analyzing data and discussing ideas. Students also do better when they work together to understand concepts and solve problems. Students who attempt to short circuit the guided inquiry process by �getting the right answer� or copying from their textbook will find that they have not really learned the material well for the homework and Problem-Solving Assessments. I will serve as a facilitator, observing and periodically addressing individual and classroom-wide needs.
Before the first class, please review the following PowerPoint slideshows that describe:
What's Up With CHEM 125? describes why we have adopted the approaches we use in class that stress teamwork in a cooperative setting and a problem-based learning approach. We will be using an "inverted classroom" in which you prepare for classes in advance through assigned readings, practice online and regular homework problems, and web-based mini-lectures. I will "lecture" for 15 minutes, but the rest of the class will consist of group problem solving.
Why do we use an "inverted" classroom and the CHEM 125 Workbook? which shows the documented advantages of our approach.
A recent article about what's better, active learning or lecture.
Most of your work will be done outside of class. Your success in the class will be determined by your effort outside of class. You may have heard a general rule of thumb that for every hour in class you should prepare at least two hours. Instead I would ask you to spend as long as it takes for you to understand the material and do the problems. Sometimes it will take more time.
Before Class: Read the assigned material (from the text or webtext). Then do the online problems on MOODLE: Chem Tutor (see below). These problems are not very difficult but will prepare you to get the most out of class;
In Class: I will divide class time into mini-lectures, group activities, and quizzes/tests. Group activities will consists of problem solving and presentations based on problems distributed in class or by email, and on activities found in the CHEM 125 Workbook. I will make groups and change them during the semester. Some classes will begin and end with a short multiple choice assessment that you can access with any smart device (phone, tablet, laptop, etc). These assessment are found at socrative.com. More information about Socrative Quizzes are found below. Please bring a personal device to each class. You will be promoted for a classroom to enter. Enter DrJChem125
After Class: Read the assigned text and web text for the next class, and a series of problems based on the learning activities of the just completed class.
Note that before the next class, you will be assigned problems based on the just completed class, and also reading material and problems to prepare you for the next class.
Most of the class will involve group work. I will construct groups which will change during the semester. Groups are smarter than any one individual within the group. In your future jobs and professions, you will never work alone. You will always work as a team. Hence it is important that you learn how to work effectively as a team. You will evaluate each member of your group as well as your own self for each group you are in during the semester.
I will use a program called Team-Maker to make groups for the semester. You will also use the program to evaluate other group members and yourself. A survey will be sent to you before we start class. It will close on Tuesday evening at 11:59 pm (second day of the semester). The groups will be ready for day 2.
Team-Maker collects information about you and your classmates to form teams according to criteria specified by your instructor. For the best teaming experience, please answer each question completely and accurately. Your responses to the survey questions are considered confidential.
SOCRATIVE QUIZZES: BEGINNING AND END OF CLASS
USE OF SAPLING: ONLINE HOMEWORK
Sapling has problems to help you learn chemistry. You will have 3 attempts to post your best score. You can always redo assignments after the due date and specified due time but it will not change your score. Don�t leave assignments until the last minute. Your first Connect assignment will be to register online and then do the �How to� assignments on the Daily Schedule for Wednesday, August 28 (due at 1:00 am that day). Here are instructions on its use.
1. Go to http://saplinglearning.com and click "US Higher Ed" at the top right.
2. If you already have a Sapling Learning account, log in then skip to step 5.
3. If you have Facebook account, you can use it to quickly create a Sapling Learning account. Click the blue button with the Facebook symbol on it (just to the left of the username field). The form will auto-fill with information from your Facebook account (you may need to log into Facebook in the popup window first). Choose a password and timezone, accept the site policy agreement, and click "Create my new account". You can then skip to step 5.
4. Otherwise, click the link "Create an Account". Supply the requested information and click "Create My Account". Check your email (and spam filter) for a message from Sapling Learning and click on the link provided in that email.
5. Find your course in the list:
o Expand the subject, "General, Organic, and Biochemistry."
o Expand the term (i.e Semester 1, Quarter 1). "Semester 1"
o Click on the link that reads your course title. " College of Saint Benedict -CHEM125- Fall14"
6. Your course requires a key code, which you will be prompted to enter. Your key code is composed of your 3-digit alphanumerical section number containing no spaces. (Example: 02B) 7. Your course requires a payment, select a payment option and follow the remaining instructions. Once you have registered and enrolled, you can log in at any time to complete or review your homework assignments. During sign up - and throughout the term - if you have any technical problems or grading issues, send an email to support@saplinglearning.com explaining the issue. The Sapling support team is almost always more able (and faster) to resolve issues than your instructor.
Help on Sapling
There is a student help page and support staff (support@saplinglearning.com) that is available to you. Here are the system requirements: xxx. Maintaining updated user settings, especially those associated with Adobe Flash, can avoid many common technical issues. If you have issues with your assignments and accessibility, please go to the help page, as it includes instructions on resolving FAQ and provides the appropriate contact information.
Student Help site: http://www2.saplinglearning.com/help/higher-education-us/student-faq
System Requirements: http://www2.saplinglearning.com/help/higher-education-us/system-requirements
Resolving Technical Issues (common method): http://www2.saplinglearning.com/help/when-i-load-my-homework-questions-are-blank-whats-wrong
For instructors: Instructor FAQ | New Instructor Orientation video | Timed Assignments | Question Weighting
Students whose primary language is not English or who have documented learning disabilities should contact me within the first two weeks of the semester to discuss possible accommodations on exams.
Help from Faulty:
You have many ways to get help with chemistry. First you should come to office hours and ask questions, especially in groups. Faculty are also available on Tuesday night, 6:30-8:00 PM at O'Connell's, a coffee house in the Haehn Campus Center which provide a relaxing atmosphere to meet and discuss chemistry. Here is the faculty tutoring schedule for Fall 2013. In addition, the following student tutors will join the faculty there.
Help from Students Tutors:
Tutors can help you with class assignments or concepts covered in the class with which you are having difficulty. A good tutor, like a good teacher, will lead you to discover the answer, often by asking questions that will lead you in the right direction. A question such as �what�s the answer for this question?� is not appropriate, nor are the tutors there to check your work before you turn it in! One way to think of the tutor sessions is simply as a place to meet to do group (or individual) assignments where there is someone available who knows what�s going on.
A CSB/SJU student supported by an NSF grant and who took CHEM 125 and 201 in the summer, will offer help sessions for our section of CHEM 125 on Monday and Wednesday evenings. A specific tutor is assigned to each section. The tutor will take attendance and assign 3 points to each student who attends. These points will be added to your homework et al grade. You are required to attend 70% of the tutor sessions to get the maximum points for this activity. If you have an ongoing conflict (e.g. an evening class or a sports practice that prevents you from spending at least a half hour at her session on multiple occasions) please contact me ASAP. You may substitute attendance at faculty tutorials (see below) or visits to my office hours for individual help. In the latter case you do not need to stay for a half hour. In these cases, get the instructor on duty to sign and date a page in your workbook that you worked on. You can then show this to me in class. At about midterm, if your overall grade is a B or better you can stop attending these sessions. You will receive the max score for this component of your grade.
There may be a few evenings when an individual tutor will not be available. The tutors are expected to post a sign at the tutoring site ahead of time when they cannot make it. There will be no tutor sessions on evenings before a free day or on days when classes are canceled due to weather.
Because of the guided inquiry approach, students need to come to class regularly in order to participate fully in their group sessions. I expect consistent, constructive, energetic yet respectful participation in group problem-solving sessions. A crucial component of participation is adequate class preparation. You have a great deal of responsibility for the success or failure of this class. Criteria for evaluating class participation will be developed in consultation with class members at the beginning of the semester.
Attendance spot checks (graded)
will occur periodically.
If a student misses more than two class periods in the time between two
Problem-Solving Assessments, the student will automatically be removed from
the team and will complete all team projects/class work on his or her own.
Your peers and/or the faculty instructor will conduct participation
evaluations regularly.
Your final grade will be influenced by the extent and quality of your participation. Prep and Participation (Moodle Tutor, Socrative, Passports, Group Dynamics/Participation) count for 10% of the grade. Significant participation might offset poorer performance on Moodle Tutor, Socrative, and Passports. Participation suggests interest and effort. It is not limited to just asking/answering questions in class. It includes things like:
The only way to really learn chemistry is to work problems.
Recent educational research indicates repetition is necessary to learn
new material. Prompt, accurate
feedback is also key to learning a new task.
Thus, there will be homework (either CONNECT problems, worksheets or
problems from the end of the chapter) due almost every day except when there is
a Problem-Solving Assessment.
CONNECT and Moodle provide instant feedback and I will try to get any graded
homework back quickly. This effort should help you to keep up with the material
and come to class with questions about the material that is being covered.
Connect and Moodle programs have specified due dates and times. Connect
and Moodle assignments are all due by 1:00 pm of the day they are assigned.
After the due date and time, you will be shut out of doing the
assignment.
These homework
assignments should keep you on track but are NOT a substitute for doing any
other problems or reviewing your notes or studying for the Problem-Solving
Assessments.
Plagiarism: I expect you to do individual work unless you are working on a group assignment. Any duplicate assignments will be considered plagiarism/academic dishonesty and will be given 0 pts for duplicates. We will do many group assignments and then I encourage collaboration within a group.
Workbook:
Do
not tear pages out of your workbook. Photocopy the page if you need to turn it
in. I will not accept assignments on
pages ripped from the workbook. We went to considerable effort to provide
this bound workbook for you so that all of your notes and problems would be in
one place. I want to encourage you to keep your materials together.
Late Assignments: Late assignments will receive
10% off per day.
Most of you probably had chemistry in high school. You may have had an introductory course, or taken AP Chemistry. You probably took it in your Junior year. You bring in differing levels of understanding of chemistry � both factual and conceptual, both of which are probably rusty. In high school, chemistry may have been one of your most difficult classes. Now, you will take chemistry again, only this time in a completely different context. For many of you in your first year at CSB/SJU, you will be taking chemistry along with three to four other demanding classes. In addition, the pace we will take will be faster than in high school. You will have to learn and use new study habits for the rigors of the academic expectations at CSB/SJU. All of this is happening at a new time in your lives when you�re trying to make new friends and adjust to a brand new environment. There will be many competing demands for your time. How are you going to deal with all of these demands? It's up to you. I can provide the learning environment, but you will have to do the learning. High grades in college don't come as easily as they do in high school. What can you do to do well?
Here is what I expect from students:
Here is what you can expect from me:
Chemistry is a difficult field. One study showed that there are as many new words in a first year general chemistry class as in a first year foreign language class. The words have precise, but often difficult meanings. Critical thinking skills are required. In addition, there is much content to learn. It's not a course that centers around discussion or your opinion, but your ability to problem solve. To be successful in the class, you will need to study regularly and diligently. Click the link below to find out some proven ways to study successfully that were compiled by Ronald Ragsdale, University of Utah..
Learning is a complex endeavor. It can be fun and easy and we would like it to be that way. More often it is challenging, difficult, and associated with failure (temporary, we hope). Our goal as life-long learners is to try new strategies when we encounter difficulties and failures on the path to understanding, and transform our fears into courage and frustrations into patience. True and deep learning comes when we overcome failures and our fears that prevent us from doing what we need to do. We move
from fearing a new challenge to having courage to face the challenge,
from frustration when our attempts fall short to patience for ourselves and the hard work we know is required
from failure to self confidence as we overcome failures.
It is easy to blame yourself or me if you have difficulty with this class. Both are unproductive. You need to adopt a new strategy, as described below. These strategies work for any situation you find difficult, not just General Chemistry.
Richard Light has interviewed 1000s of students to determine how students can maximize their learning at colleges and universities. He has identified several key principles. One is that Interactive relationships organized around academic work are vital. Successful students usually have one or more "intense relationships built around academic work". He suggests that students join a small study group outside of class. Our extensive use of group collaborative work in class is consistent with this model. Time management skills are very important. Lack of time management skills and plans is associated with academic problems. He notes that first year student often have trouble dealing with the amount and speed of academic work they encounter in their first year. Many are not prepared for the transition. Success requires that students make intentional use of time. For example, he advises students to divide the day into three parts, morning, afternoon, and evenings, and to try to use one for uninterrupted focused academic work. Also he suggests that students be intentional about use of gaps between activities. When you study, do it intensely and in an uninterrupted fashion.
Psychologists tell us the best way to study: Scientific American
WHAT DOES IT TAKE TO GET AN A?
Sending any of the course materials from any course at CSB/SJU to a third party vendor is expressly forbidden and will carry serious consequences.
I have the right to amend this syllabus during class in ways which I feel will facilitate student learning.
Last Update: 09/09/2014