Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
B: OXIDATIVE ENZYMES
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8B: After class and this reading, students will be able to
|
B2. The Chemistry of NAD+ and FAD
NAD+ is a derivative of nicotinic acid or nicotinamide.
Figure: NAD+ is a derivative of nicotinic acid or nicotinamide.
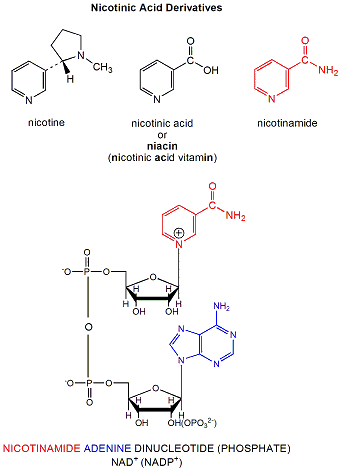
It and its reduction product, NADH, exists in the cells as interconvertible members of a pool whose total concentration does not vary significantly with time. Hence, if carbohydrates and lipds are being oxidized by NAD+ to produce energy in the form of ATP, levels of NAD+ would begin to fall as NADH rises. A mechanism must be be present to regenerate NAD+ from NADH if oxidation is to continue. As we will see later, this happens in the muscle under anaerobic conditions (if dioxygen is lacking as when you are running a 100 or 200 m race, or if you are being chased by a saber-toothed tiger) when pyruvate + NADH react to form lactate + NAD+.
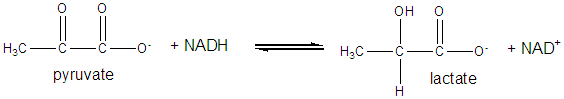
Under aerobic conditions (sufficient dioxygen available), NADH is reoxidized in the mitochondria by electron transport through a variety of mobile electron carriers, which pass electrons to dioxygen (using the enzyme complex cytochrome C oxidase) to form water.
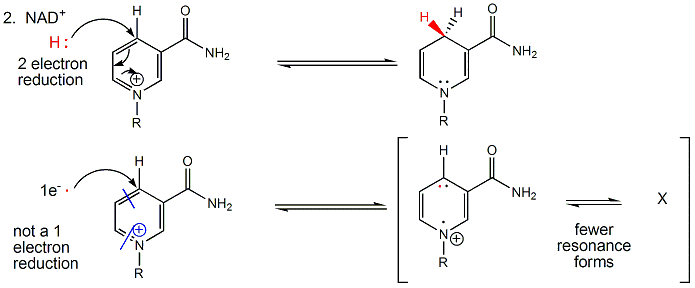
NAD+/NADH can undergo two electron redox steps, in which a hydride is transferred from an organic molecule to the NAD+, with the electrons flowing to the positively charged nitrogen of NAD+ which serves as an electron sink. NADH does not react well with dioxgyen, since single electron transfers to/from NAD+/NADH produce free radical species which can not be stabilized effectively. All NAD+/NADH reactions in the body involve 2 electron hydride transfers.
Figure: All NAD+/NADH reactions in the body involve 2 electron hydride transfers
FAD (or flavin mononucleotide-FMN) and its reduction product, FADH2, are derivatives of riboflavin.
Figure: derivatives of riboflavin
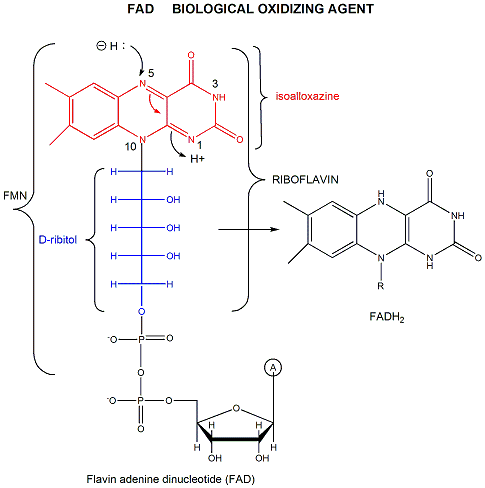
FAD/FADH2 differ from NAD+/NADH since they are bound tightly (Kd approx 10-7 - 10-11 M) to enyzmes which use them. This is because FADH2 is susceptible to reaction with dioxygen, since FAD/FADH2 can form stable free radicals arising from single electron transfers. FAD/FADH2 can undergo 1 OR 2 electrons transfers.
Figure: FAD/FADH2 can undergo 1 OR 2 electrons transfers
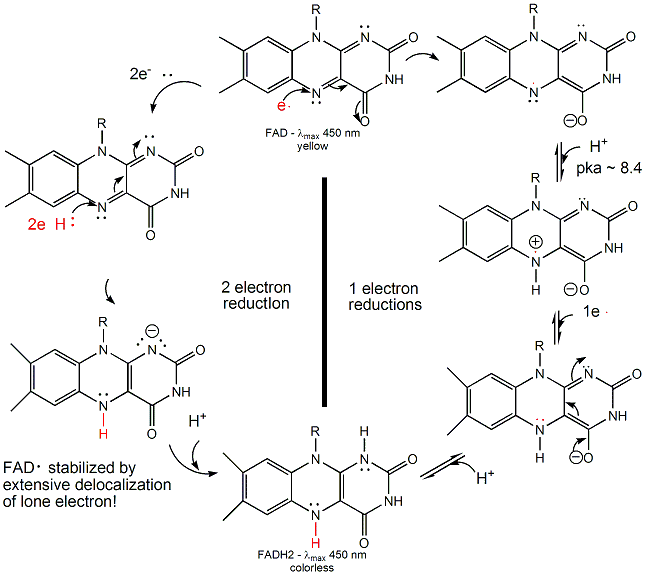
FAD/FADH2 are tightly bound to enzymes so as to control the nature of the oxidizing/reducing agent that interact with them. (i.e. so dioxygen in the cell won't react with them in the cytoplasm.) If bound FAD is used to oxidize a substrate, the enzyme would be inactive in any further catalytic steps unless the bound FADH2 is reoxidized by another oxidizing agent.
Navigation
Return to Chapter 8B: Oxidative Enzymes Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8B: Oxidative Enzymes

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.