Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
B: OXIDATIVE ENZYMES
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8B: After class and this reading, students will be able to
|
B7. Dioxygenases
An example of a dioxygenase is the cyclooxygenase activity of prostaglandin synthase. This enzyme, often just called cycloxygenase or COX, is an integral membrane protein found in the ER membrane, and is a homodimer (with two hemes). It catalyzes two different reactions. One is the addition of two dioxygens to arachidonic acid - 20:4Δ5, 8, 11, 15(which is liberated from the C2 position of phospholipid membranes by phospholipase A2 upon appropriate signaling) to form prostaglandin PGG2. This molecule, with 5 chiral centers, arises from arachidonic acid, which has one. The cyclooxygenase activity is buried in the membrane, from where the arachidonic acid can readily access its active site. The active site is at the end of a hydrophobic channel (arachidonic acid binding site) and stretches from the membrane-binding region to a buried heme. PGG2 can be further metabolized to PGH2 by the addition of two electrons to PGG2 by the hydroperoxidase activity of the enzyme, located at the other end of the enzyme. This activity forms an alcohol from the peroxide functional group in PGG2. There is one heme per momomer, which acts in both the cyclooxygenase and peroxidase activities. Each monomer of the dimer has both enzymatic activities.
Figure: Arachadonic Acid Bound to the Active Site of Mouse Cyclooxygenase
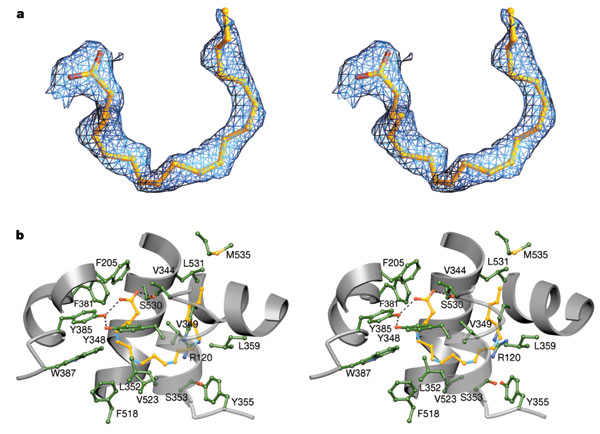
Reprinted by permission from Nature
Kiefer et al. Nature 405, 97-101
(2000)
Nature
Arachidonate and prostaglandin bound to the cyclooxygenase active site of wild-type COX-2. a, Stereo image of the experimental difference electron density map before the inclusion of AA (green trace) or PGH2 (gold trace) b, Diagram of PGH2 (gold) and AA (cyan) bound to the cyclooxygenase active site. Mobile side chains are colored gold and cyan to match their PGH 2 and AA bound positions, respectively.
Notice the hydrophobic nature of the amino acids surrounding the arachidonic acd. Note as well the kinks in the arachidonic acid associated with 4 all-cis double bonds in the fatty acid.
The reaction mechanism appears to involve a free radical. A possible free radical cascade mechanism, based on recent work by Marnett et al. and Kiefer et al. is shown and summarized below. Note that amino acids important for catalysis listed below do not appear be in the same positions as shown in the figure above. Mutagenesis clearly shows the importance of Tyr 385 in catalysis. The crystal structure above represented an alternative binding mode for arachidonic acid/COX2 that would not be catalytically active.
- The carboxylate of arachidonic acid is coordinated to Arg 120 and Tyr 355.
- The C13 pro(S) H atom of arachidonic acid is close to Tyr 385 which allows its abstraction.
- This results in a radical centered on C11 that reacts with dioxygen to form a peroxyl radical
- Attack by dioxgen at C11 occurs from the side of the substrate opposite to that of hydrogen abstraction
- The oxygen radical at C11 cyclizes by attacking C9.
The C13 proS hydrogen atom (not proton) is remove from bound arachidonic acid by a free radical form of Tyr 385, which acts as an oxidizing agent. A site-specific mutants in which Tyr 385 is replaced by Phe is inactive. How is the Tyr free radical formed? Based on single electron standard reduction potential (0.9 V for Tyr and -0.2 to + 0.2 V for Fe3+ in the bound heme), it appears that the heme iron is not a potent enough oxidizing agent to accomplish this task. However, oxygen bound to the heme iron could be converted to a peroxide and form an Fe4+-oxo complex much like we saw step 7 of a possible reaction pathways for cytoP450cam. The Fe4+ ion is a more potent oxidzing agent (standard reduction potential of approximately 1V, sufficient for oxidation of Tyr 385. Another possibility is that the peroxide activator (in the formation of the ferryl-oxo ligand) is NO (nitric oxide, a free radical). NO is formed by immune cells (like macrophages) on immune activation. The NO might react with superoxide (also a radical, possibly formed during an oxidative burst in macrophages during immune stimulation) to form peroxynitrite (NO3-). This can donate an oxo group to the Fe3+ to form the Fe4+-oxo complex, which could then oxidize Tyr to the free radical form. There might be other mechanisms as well to generate the Tyr free radical, since just adding organic peroxides to the enzyme will generate it.. After abstraction of the proS H atoms, a carbon-centered free radical at C11 results which reacts with oxygen as shown below. The exact form of oxygen that reacts is unclear, but presumably is either a peroxy or activated singlet form.
Figure: Prostatglandin Synthesis: Possible Mechanism
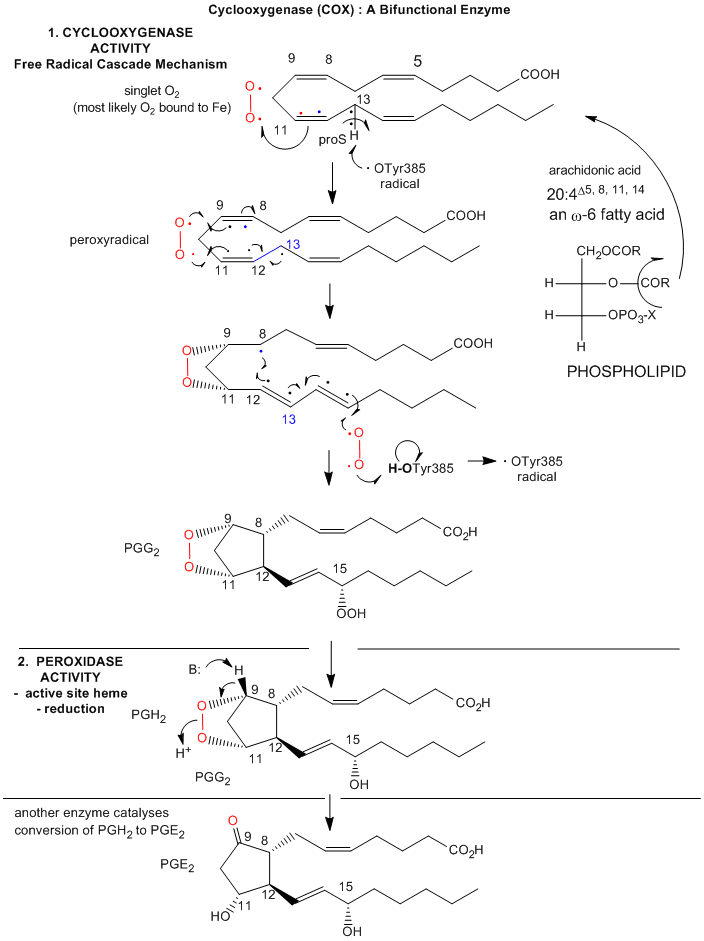
Prostaglandins, which were first isolated from prostate glands, serve as powerful, but labile local hormones which are mediators of pain, inflammation, immune and clotting activity. The cyclooxygenase activity is inhibited by aspirin, which probably accounts for most of its anti-inflammatory and analgesic properties. Aspirin, acetylsalicylic acid, acetylates a reactive Ser 530 in the active site. Another nonsteroidal anti-inflammatory drug (NSAID) with similar properties is Ibuprofen (Advil). Acetaminophen (Tylenol) is also considered a member of this drug class, even though it doesn't have anti-inflammatory properties. The question has arisen as to why. It now turns out that there are apparently three different types of COX, I, II, and III. COX III is expressed in the brain, and might be involved in pain pathways. Acetaminophen appears to work on this COX, as shown in the chart below (Bazan et al.).
Cyclooxygenase Activities
| COX | Expression | Function | Inhibitors |
| COX 1 | constitutively | organ pain, platelet function, stomach protection | NSAIDs including aspirin |
| COX 2 | induced by growth factors, neurotransmitters, inflammatory cytokines, oxidative stress, injury. Constitutively in brain, kidney | Inducible COX2: inflammation, pain, fever Constitutive COX2: synaptic plasticity |
NSAIDs, COX 2 inhibitors including celecoxib (Celobrex ) which has few GI problems associated with its use |
| COX 3 | constitutively, high in brain, heart | pain pathways, not inflammation pathways | acetaminophen (no GI problems, great fever reducer), some NSAIDs |
![]()
![]() Jmol:
Cyclooxygenase I and II
Jmol:
Cyclooxygenase I and II
Fish n-3 fatty acids and health
We mentioned the importance of arachidonic acid in signal transduction in the lipid chapter. In addition, the importance of n-3 fatty acids to health was discussed as well. As mentioned above, arachidonic acid is cleaved from the C2 or sn-2 position of membrane phospholipids and modified by cyclooxygenase or lipoxygenase to form prostaglandins and leukotrienes, both potent local biological mediators. Linoleic acid and 22:6n-3 (DHA or docosohexaenoic acid) are also found in membrane phospholipids at the sn-2 position. What is the mechanism for the health-protective effects of n-3 fatty acids like DHA?
In human tissue, DHA, 22:6n-3 or 22:6Δ5,8,11,14,17,20 is the most abundant n-3 polyunsaturated fatty acids (PUFAs). Since it is synthesized from linolenic acid (as is EPA), a deficiency of linolenic acid in the diet will lead to lowered levels of 22:6n-3 in tissues, with ensuing health effects. Since these lipids are involved in membrane structure, signal transduction, and hormone synthesis, diverse effects of dietary n-3 PUFA deficiency will be observed. 50% of all fatty acids in the sn1 and sn2 position of membrane phospholipids of rod outer segments (in the retina) are 22:6(n-3). Cognitive dysfunctions (loss of memory, etc.) have been linked to decreased levels of 22:6(n-3) in the brain. This fatty acid binds to retinoid X receptors which then activate (through linked binding reactions) nuclear receptors, leading to alterations in gene transcription in the CNS.
In other tissues, 22:6(n-3) rarely exceeds 10% of membrane fatty acids, but this percentage can be increased in cells with increases in a precursor, 20:5(n-3). DHA might affect lipid rafts in the membrane, which would affect movement of important membrane protein receptors (and associated proteins) in the membrane, altering cell response to environmental stimuli. DHA and EPA affect arachadonic acid conversion to prostaglandins and leukotrienes. EPA binds less tightly to cycloxygenase I and is a poor substrate for the enzyme, both effects which inhibit the formation of prostaglandins and signaling processes mediated by them. This explains why n-3 fatty acids have anti-inflammatory effects.
In addition, n-3 fatty acids have noticeable effects on gene transcription, which remain as long as these fatty acids are present in high levels in the diet These and other fatty acids bind to fatty acid-activated transcription factors called PPARs (peroxisome proliferator receptors - alpha, beta and gamma 1 and 2). These receptors regulate, through alterations in gene expression, proteins involved in lipid metabolism. Other fatty acid-dependent transcription factors are known as well. PPAR's bind 20:5(n-3) with a micromolar Kd and change the conformation of the protein to a form than can bind other proteins, ultimately altering gene expression.
Table: Biological Effects of n-3 polyunsaturated fatty acids EPA (20:5) and DHA (22:6)
| Organ(s) | Effect | Mechanism acts through |
| central nervous system | improve cognitive function | membrane composition; retinoic X receptor alpha |
| retina | improve acuity | membrane composition |
| immune | immunosuppressive; antinflamatory | membrane composition; rafts |
| cardiovascular | anti-arrhythmia; anti-clotting |
membrane composition; rafts; eicosanoids |
| serum lipids | lowers triglycerides (risk factor for cardiovas. dis) | peroxisome proliferator receptor alpha and gamma |
| liver | decrease lipid synthesis; increase fatty acid oxid. decrease VLDL synthesis |
sterol reg. element bind. protein; PPAR alpha PPAR alpha |
Adapted from Jump D. The Biochemistry of n-3 Polyunsaturated fatty acids. J. Biol. Chem. 277, pg 8755 (200)
Navigation
Return to Chapter 8B: Oxidative Enzymes Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8B: Oxidative Enzymes

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.