Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
B: OXIDATIVE ENZYMES
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8B: After class and this reading, students will be able to
|
B9. Heme Proteins
So far in this course, we have examined three different kinds of heme proteins..
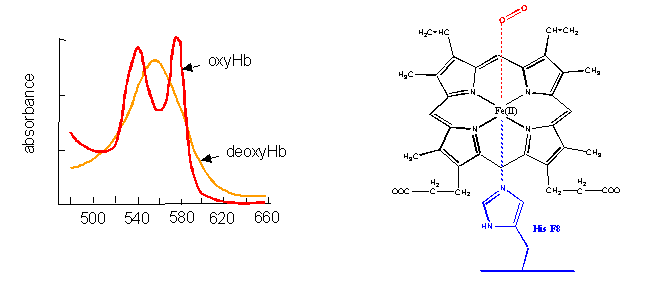
- The first, hemoglobin (and myoglobin) serve as carriers of dioxygen. Even though they bind one of the best oxidizing agents around (dioxygen), the heme Fe2+ does not get oxidized to Fe3+. If it does, as in the case of met-Hb, the protein looses it ability to carry oxygen.
Figure: hemoglobin (and myglobin) serve as carriers of dioxygen
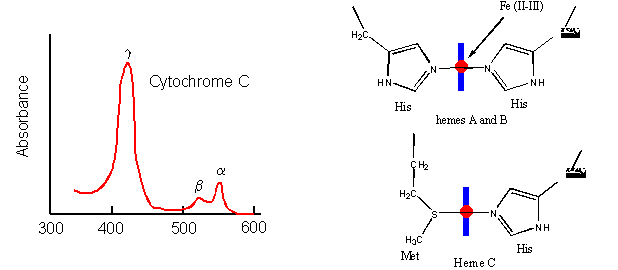
- Cytochrome C, on the other hand, does not bind dioxygen but rather serves as a carrier of electrons which get passed to dioxygen in Cytochrome C oxidase. Its Fe ion readily cycles between the 2+ and 3+ states as it serves as an electron carrier.
Figure: Cytochrome C
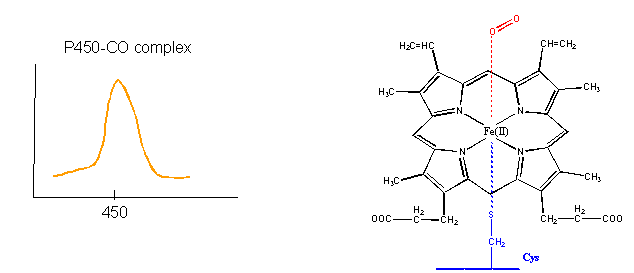
- Finally, the Fe2+ in the heme of the cytochrome P450s (so named since they have an absorbance maximum at 450 nm when they bind CO) does both. It binds dioxygen and cycles between the 2+ and 3+ states as it activates dixoxygen for hydroxylation reactions.
Figure: cytochrome P450s
How could heme serve such diverse functions? We can explain this by referring to one of the main themes of the course - structure mediates function. The environment of each heme must be different. Clearly the protein ligands coordinating the Fe ions are different. The 5th ligand is the proximal His in hemoglobin while dioxygen binds to the 6th site. In cytochrome C, the 5th and 6th ligands are His and Met, respectively. In cytochrome P450, the 5th site is occupied by Cys, and the 6th by dioxygen. Presumably the environments surrounding the hemes are different as well. Once again, we have seen analogous example in which chemical properties are influenced by the microenvironment. The pKa of a given amino acid side chain can vary considerably depending on the polarity of the local environment. Likewise, the standard reduction potential of tightly bound FAD/FADH2 depends on the microenvironment.
As we have seen (from the study of heme proteins and the oxidative enzymes of cells), transition metals such as Fe, Zn, and Cu have vital biological roles as binding sites and cofactors in many reactions. Yet they also pose problems since they can lead to oxidative damage in cells. As we saw with cytoplasmic metallothioniens, which bind to heavy metals and protect the cell from such damage, many proteins are involved in binding and regulation of transition metals in the cell. Integral membrane proteins are required to bind and transport these cations into the cytoplasm. Other proteins act as sensors of transition ion concentration (such as latent transcription factors which bind heavy metals and become active transcription factors for metallothioniens. Others act as chaperone proteins which bind metal ions and transfers them to apometalloproteins. Recent work has suggested transporters and chaperones involved in metal ion biology bind these ions with unusual coordination geometry, which presumably facilitates transfer of the ion to the apo-target protein.
The transition metals Zn and Fe are often found in E. Coli at a concentration of 0.1 mM, compared to Cu and Mn which are present at concentrations from 10 to 100 μM. Also, about one third of all proteins demonstrate specific binding of metal ions and can be classified as metalloproteins. Mass balance suggests that metal ions would be distributed in proteins with low, intermediate, and high metal binding affinity as well as in free pools, which which potentially be toxic to cells. Metalloproteins, depending on their Kd for metal ion binding, would hence be in various state of ligation. The free concentration of some ions (Cu and Zn) is so low that newly synthesized apoproteins which bind these ions would not obtain the ion from the free pool. In such cases, metal chaperones would be required.
Navigation
Return to Chapter 8B: Oxidative Enzymes Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8B: Oxidative Enzymes

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.