Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
C: ATP AND OXIDATIVE PHOSPHORYLATION
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8C: After class and this reading, students will be able to
|
C12. Metabolic Needs in Nonproliferating and Proliferating Cells
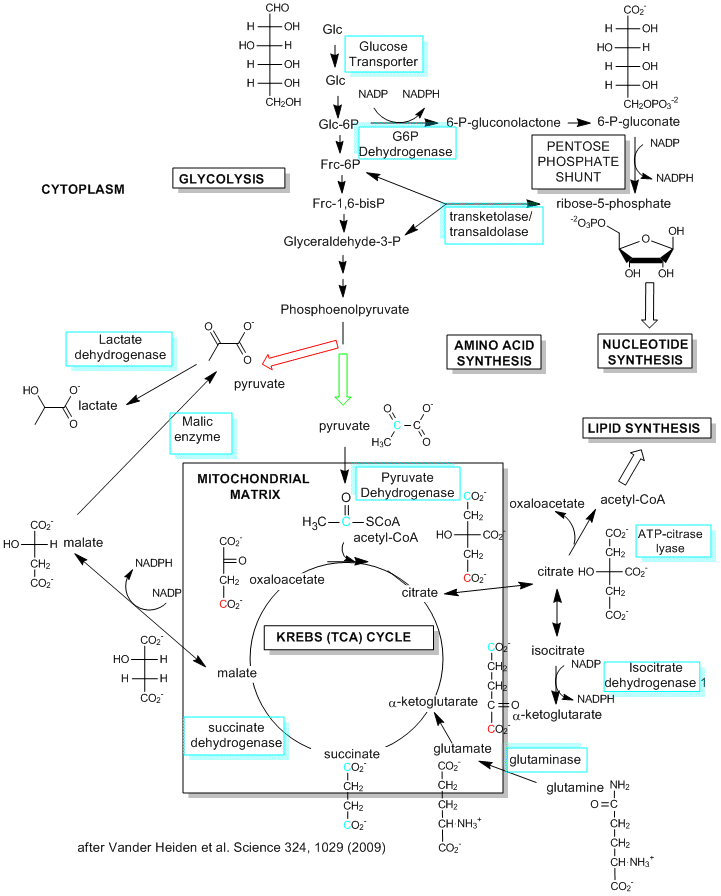
Although most of this chapter deals with the extraction of energy from glucose through the production of ATP in glycolysis (anaerobic) and the TCA cycle and oxidative phosphorylation (aerobic) in the mitochondria, cells have other needs that must be met, especially the need for reductive biosynthesis to produce fatty acids, proteins, and nucleic acids. Which need prevails? Vander Heiden et al, in a recent review, suggest that it depends on the metabolic needs of the cell. Our understanding of metabolic pathways and their control derive mostly from the study of nondividing cells that are terminally differentiated. In these cells, the need for reductive biosynthesis is minimal so cells extract energy most efficiently from glucose through aerobic oxidation of the glycolytic end product, pyruvate, through mitochondrial oxidative phosphorylation. What about cells that are actually dividing and differentiating? They argue that these cells have a great need for reductive biosynthesis (think of the need to duplicate the contents of the entire cell, which effectively increases the biomass). This would mandate that cells maximize the production of small molecule precursors for synthesis of larger molecules. These small molecule precursors include acetyl CoA for the synthesis of fatty acids for membranes, glucose-6-phosphate for the synthesis of ribose and deoxyribose for the synthesis of RNA and DNA, and a myriad of small molecules or additional metabolites arising from glycolysis and the TCA cycle. If these small molecules, which are produced in the cytoplasm or move from the mitochondria to the cytoplasm, are removed from the energy producing pathway for reductive biosynthesis, how does the cell meet its energy needs?
One type of proliferating cell that has been well studied is a tumor cell. These cells, which have great need for reductive biosynthesis, have long been know to undergo aerobic glycolysis, and in the process produce large amounts of lactate. This effect was observed by Warburg in 1956, who thought the effect arose through defective mitochondria in tumor cells (which is not the case). Proliferating, single cell organisms also engage in aerobic glycolysis. It now appears that proliferating, non-tumor cells from multicellular organisms do as well. Aerobic glycolysis (glycolysis in the presence or absence of dioxygen) would obviously occur most readily under adequate nutritive conditions which would be present in multicellular organisms receiving a constant stream of nutrients delivered by the blood. Under restrictive nutrient conditions, cells would undergo cell phase arrest to minimize proliferation. In contrast, differentiated and nonproliferating cells from multicellular organisms, with no need for significant reductive biosynthesis, would obtain energy most efficiently through mitochondria aerobic pathways.
A little stoichiometry will clarify the differing needs required by the pathway which leads to the most efficient use of glucose for ATP production (through converison to pyruvate and its continued conversion of carbon dioxide aerobocially) and use of glucose for reductive synthesis of the palmitic acid (16:0). The net equation for the production of 16:0 from metabolites of glucose (acetyl CoA formed from progression of glucose to pyruvate in glycolysis and its oxidative subsequent oxidative decarboxylation to acteyl CoA in the mitochondria) is:
8 CH3(CO)SCoA + 7 ATP + 14 NADPH --> 1 palmitic acid + 7 ADP + 7 Pi + 14 NADP+
(Note: NADPH is a phosphorylated version of NADH found in the cytoplasm and is used for reductive biosynthesis instead of NADH. Reduced nicotinamide adenine dinucleotide molecules needed for reductive biosynthesis are differentiated from those produced during catabolism by being phosphorylated and by being predominantly found in a different cellular compartment (the cytoplasm compared to the mitochonria).
From where do all the needed ATP and NADPH molecules derive? Lets consider that question starting from glucose.
-
1 Gluose produces around 36 ATPs from mitochondrial TCA cycle followed by oxidative phosphorylation.. Hence on a molar ratio basis, 0.2 mol mol of glucose are required to produce the necessary 7 mol ATP for synthesis of 1 mol of 16:0. .
-
1 Glucose produces 2 NADPH when the first product in glycolysis, glucose-5-phosphate is withdraw from glycolysis and moved into an altenrative pahway, the pentose phosphate shunt, that produces ribose 5-phosphate for RNA and DNA production. Hence 7 mol glucose (35x the amount needed to make the required ATP) are needed to produce the required 14 mol of NADPH required to produce 1 mol of 16:0.
-
4 Glucose molecules produce 8, 2C-CH3(CO)SCoA (with the other 8 carbons atoms originally in 8 pyruvates lost as CO2 on conversion to acetylCoA by pyruvate dehydrogenase).
Hence there is greater molar need for glucose to be use for production of the small molecule intermediates necessary for 16:0 reductive bioynthesis in proliferating cells than the molar need for glucose to produce the energy (in the form of ATP) required for 16:0 synthesis. This suggests that targeting important but to some "boring" enzymes involved in glycolysis and the TCA cycle (which to reiterate are the sources of the small molecule precursors for reductive biosynthesis) might inhibit tumor proliferation.
Proliferating cells have high ATP/ADP and NADH/NAD ratios which leads to feedback inhibition of important steps in energy production, including the synthesis of citrate from oxaloacetate and pyruvate in the first step of the TCA cycle. Under these conditions, citrate leaves the mitochondria where it cleaved in an ATP depended fashion back into acetyl CoA (which can now be used for fatty acid synthesis) and oxaloacetate. To allow continue TCA activity, glutamine, an amino acid which is also metabolized in high quantities in proliferating cells, can be converted in the mitochondria to glutamic acid which after loss of ammonia forms alpha-ketoglutarate, an intermediate in the TCA cycle. This allows continued energy production in the TCA cycle.
How much of glucose is used for energy production versus small molecule precursor productions. Vander Heiden at al suggest that in proliferating and tumor cells, about 85% is used in lactate production, and 5% used in mitochondrial oxidation, while 10% is shunted for precursor production. 60% of glutamine is also used (as described above) for biosynthesis. It would appear that lactate that results from this process is wasteful of carbon atoms that could go into reductive biosynthesis. However, it can be recycled through the Cori cycle, in which the liver converts it into glucose which is exported.
Figure: Metabolic Pathways in Proliferating Cells
Navigation
Return to Chapter 8C: ATP and Oxidative Phosphorylation
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8C: ATP and Oxidative Phosphorylation

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.