Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
C: ATP AND OXIDATIVE PHOSPHORYLATION
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8C: After class and this reading, students will be able to
|
C8. Overall Coupling Oxidation and ATP Synthesis
How is this proton gradient coupled to ATP synthesis? Another mitochondrial inner membrane complex, FoF1ATPase, also called ATP synthase or complex V, is found in the inner mitochondrial membrane.
Figure: FoF1ATPase, also called ATP synthase
It contains two domains, a transmembrane proton channel, and a enzymatic domain which can either synthesize or hydrolyze ATP. As protons stream through the membrane pore, conformational changes, probably mediated by concerted changes in amino acid side chain pKas. cause the protein to synthesize ATP. Based on kinetic and structural data, Boyer devised an innovative hypothesis for the mechanism of ATP synthesis, which has been verified by structural data. In this model the enzyme, which has multiple subunits, has 3 sites for ATP binding, named L, O, and T. The L or Loose site, binds ATP loosely, the T or Tight site binds it tightly, while the O or Open site does not bind ATP. Although the ΔGo for ATP synthesis in solution is +7.5 kcal/mol, it appears the ΔGo for bound ADP + Pi ----> bound ATP is about 1. Hence the reaction is readily reversible. The difficulty lies in dissociating the bound ATP from the complex. Initially, ADP and Pi bind to the L site. A conformational change occurs, switching the site from L to T, and concomitantly, a T site with ATP bound to an O site which promotes ATP departure. Since the T site has ADP and Pi bound, but has high affinity for ATP, it promotes the synthesis of ATP at that site. This reflects the idea that enzymes bind the transition state (which presumably looks more like ATP than ADP and Pi) more tightly than the substrate. ADP and Pi bind to the newly formed L site which promotes the switch from the T to O site, releasing ATP from the enzyme.
It should now be clear why the enzymes for oxidative phosphorylation in aerobic conditions are membrane bound. Only in this way could a proton gradient be established. Protons must be vectorially transferred in one direction only for a gradient to be established!
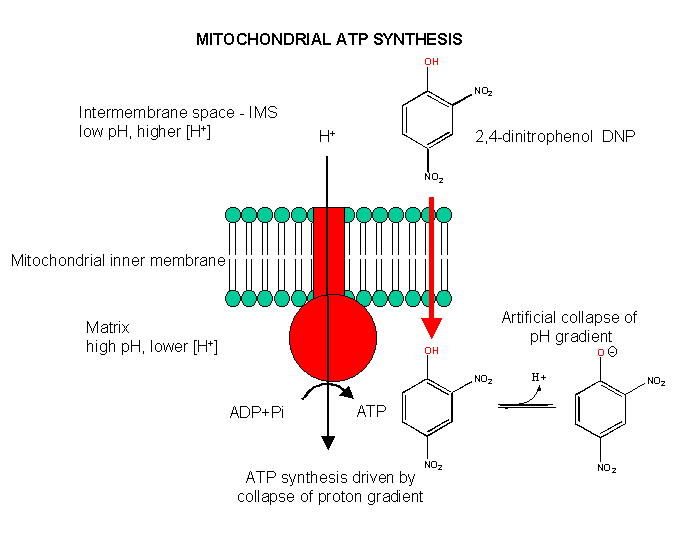
Aerobic ATP production can be uncoupled from electron transfer, as we saw with arsenate uncoupling of ox/phos in aneraobic metabolism in glycolysis. In that case, the energy sources driving ATP synthesis was removed through hydrolysis of the mixed carboxylate/arsenate anhydride. In aerobic metabolism, the energy source is the proton gradient. If this gradient could be artificially collapsed, ATP synthesis would stop, but electron transport (oxidation of NADH through formation of water from dioxygen) would continue. 2,4-dinitrophenol can collapse the proton gradient and act as an uncoupler. In the low pH milieu of the intermembrane space, this weak acid would be protonated. It is also sufficiently nonpolar so as to have reasonable bilayer permeability. When it reaches the higher pH matrix, it can deprotonate. The net effect is to shunt protons through the intermembrane and not through the F0F1ATPase.
Figure: Uncoupling Aerobic Ox/Phos
Navigation
Return to Chapter 8C: ATP and Oxidative Phosphorylation
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8C: ATP and Oxidative Phosphorylation

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.