Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
B: NEURAL SIGNALING
BIOCHEMISTRY - DR. JAKUBOWSKI
06/10/14
|
Learning Goals/Objectives for Chapter 9B:
|
B12. Touch
A theme of this course has been that binding interaction initiate biological activity. These include substrate binding to enzymes, transcription factors binding to other proteins and DNA, and signaling molecules binding to membrane receptors. These interactions allow free or membrane-bound macromolecules to "sense" their microenvironment. Odorants and tastants also initiate biological responses by binding to membrane receptors (usually serpentine receptors coupled to G proteins). Sight, another sense, is initiated by "binding" (of course I mean interaction with) a photon. But what about touch? Kung has recently written a review to discuss mechanisms involved in mechanosensation, which is ubiquitous in the biological world. He gives many examples in which a biological response is initiated by "touch", including these more uncommon examples:
- worm and paramecium movement,
- plant root response to gravity (gravitropism)
- ratio of height to girth growth in stems to wind and rain (thigmomorphogenesis),
- blood pressure changes (animal baroreceptors),
- muscle stretching (spindle receptors),
- limb position (proprioreceptors)
- texture (receptors in tongue),
- hearing, and
- osmotic pressure changes.
First we will consider the more studied mechanosensitive (MS) ion channels, which respond to changes in turgor induced by osmotic changes in bacterial cells. When reconstituted in purely lipid bilayer vesicles, these proteins can detect forces applied to the membrane. Analogous receptors have been found in animals cells as well. These channels appear to detect changes at the interface between the protein and lipid, and are probably lipid-gated channels.
Consider changes in a bacterial cell placed in pure water. Influx of water into the cells produces a turgor in the cell that causes a great force on the membrane. Cells response by releasing small ions and molecules from the cell (95% of free Pro, for example). Membrane protein involved in this process are called mechanosensitive channels of large and small conductance (MscL and MscS). These channel proteins are nonselective (compared to usual ion channels which demonstrate ion specificity). They retain activity (force induced permeability) in liposomes even if only one type of lipid constitutes the membrane.
Since the lipid bilayer, compared to the bulk solution, is highly ordered (or anisotropic) in an directional sense, it displays a gradient of physical properties at different depths into the membrane. As water displays a significant surface tension (since hydrogen bond-induced forces on water differ for bulk interior water and surface water), so does the bilayer have a significant surface tension between the polar head groups and nonpolar tails. These forces are counterbalanced by other to produce a thermodynamically stable bilayer. Membrane proteins imbedded in the bilayer would experience these forces, and change in interfacial lipids around the protein would be expected to alter the forces on the protein and provide a mechanism for lipid gating of the channels. Steered molecular dynamic simulations (in the sense that forces can be applied at specific locations in a protein) of the MscL imbedded in a bilayer have shown that force channels applied on the protein at the headgroup/acyl chain interface caused a transient opening in the channel. Translational or flip-flop diffusion of lipids to the channel/lipid interface, or addition to the membrane of hydrophobic molecules, such as anesthetics, or amphiphilic molecules, such as phosphatidyl choline with one fatty acid remove or arachidonic acid (both of which can arise in signal transduction processes) can induce opening as well. Gating of the channel appears to require changes in lipid composition in just one leaflet, which would induce localized shape changes such as bulges or cavitations that would alter membrane forces.
Similar proteins have been found in plants and animals. The mammalian TREK-1 potassium channels can be opened by trinitrophenol (an amphiphathic bulge former or crenater) and closed by chloropromazine (an amphipathic cavity-former or cavitator). Single chain amphiphiles like lysophosphatidic acid, which can be modeled like a cone, is an activator (by crenation). Many anesthetics activate it as well. Although structural cytoplasmic protein scaffolding (the cytoskeleton) may be associated with these membrane channels, their involvment in force change in the channel protein and concomitant opening/closing of the channel may not be necessary. Another set of similar proteins, transient receptor potential (TRP) proteins, which sense vibration and other stimuli, have been recently studied. There appears to be many subfamilies of these proteins. Sensory hair cells, responsible for sound transduction, appear to transmit force to a TRP protein through a tether, presumably protein constituents of the cytoskeleton.
Kung argues that two types of cell membrane sensors were required in early cells, those that recognize changes in solute concentration through a "lock and key" mechanism, and those that recognize changes in solvent concentration, which would occur under conditions of excess (rain) and depletion (drought). These changes would be transmitted to membrane sensors not by a lock and key binding mechanism (given the small change in water concentrations from the pure state of 55.5 M, but by osmotic pressure changes affecting the turgor of the cell.
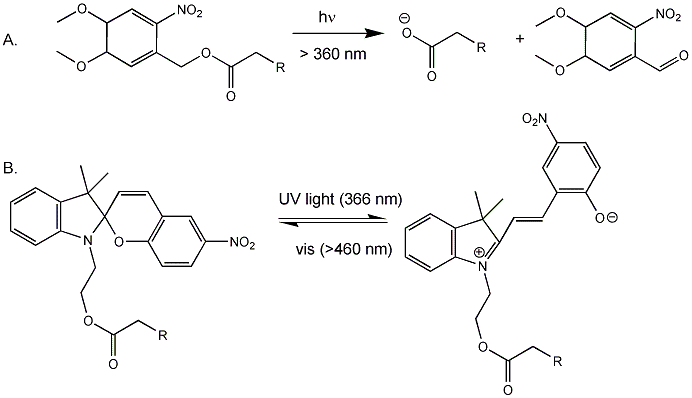
Back to the bacterial MscL for a moment. It consists of 5 identical subunits, each with 2 transmembrane alpha helices, M1 and M2. The pore is lined with the M1 subunits from each subunit. Mutagenesis or covalent modification of glycine 22 in the M1 helix to a polar or charged substitutent causes increase in pore size and hydration in the pore. Kocer et al changed the Gly to a Cys (no other Cys are found in the protein). Then they modified the Cys with an photosensitive ester analog of iodoacetic acid (Figure A below, where R = I). When illuminated with long UV (366 nm), the ester cleaves (photolysis) to form a negatively charged carboxylate and a product with a polar end near the carboxylate. This induced charge separation mimics conformational changes induced in the protein during osmotic shock, causing an opening in the pore when reconstituted into artificial bilayers. The photolysis is irreversible. They then tried another modification (Figure B below) in which the attached molecule is not photolyzed into two separate molecules, but reacts through ring opening to a form with separate + and - charges. This species could be irradiated with visible light and return to the original structure. They effectively made a "light-actuated" nanogate or nanovalue in the channel which allowed flux of ions across the membrane. To test the usefulness of this nanoengineering feat, they made liposomes with a fluorophore inside and the light-gated modified MscL protein in the membrane. The encapsulated fluorophore in the liposome self-quenches when present at high concentration. Upon irradiation, the pore opened as reflected by increased fluorescence when fluorophore levels inside the liposome decreased, which reducing fluorescence quenching.
Figure: Light-Activated Nanovalve in MscL
![]() Movie:
Steered MD simulation of MscL
Movie:
Steered MD simulation of MscL
Navigation
Return to Chapter 9B: Neural Signaling Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9B: Neural Signaling

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.