Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
B: NEURAL SIGNALING
BIOCHEMISTRY - DR. JAKUBOWSKI
06/10/14
|
Learning Goals/Objectives for Chapter 9B:
|
B6. Ligand-Gated Acetylcholine Receptor of the Neuromuscular Junction
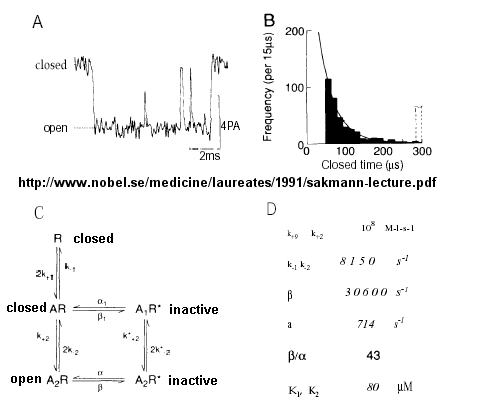
Kinetic modeling based on patch-clamp
recordings show that the protein can exists in multiple conformations,
including a closed (C), open (O), and inactive (I) forms. The binding of two
acetylcholine (A) molecules are required to open the channel. When two
are bound, the protein exist in two interconvertible forms: A2C <==> A2O.
Through another conformation change, the open form O can inactivate in a
slow step to A2I, where I is an inactive form.
The channel consists of
five helices which are constricted by hydrophobic side chains which point
inward into the pore. A rotation and sliding of the helices relative to each
other probably occurs when ligand binds the protein, allowing the channel
opening to increase. Rings of negatively changed side chains line the pore
at the top and bottom accounting for the selectivity of the channel for
positive ions.
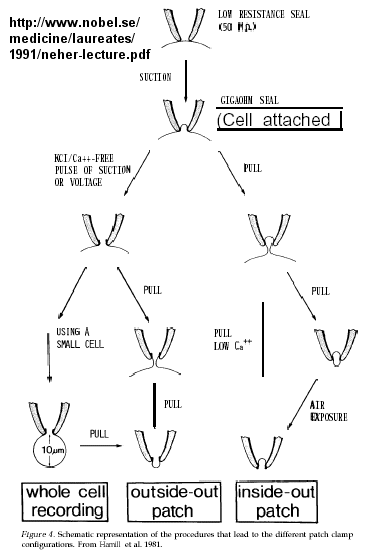
Much of our understanding of the mechanism of the acetylcholine channel opening comes from a patch clamp technique in which a micropipette is used to remove a small section of a membrane containing a channel.
Figure: patch clamp technique
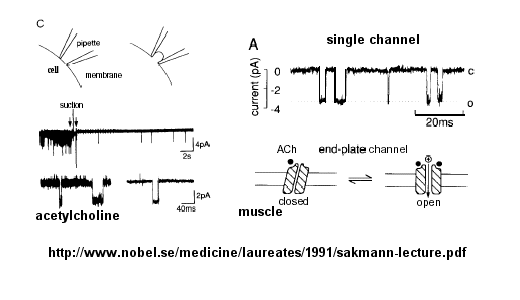
Ion flow, measured as picoamp current, can be measured across the membrane patch. The opening and closing of single channels can be recorded using this technique, for which Nehert and Sakman won the Nobel Prize.
Figure: opening and closing of single channels
From this, they deduced a mechanism for gating of the acetylcholine channel.
Figure: mechanism for gating of the acetylcholine channel
In general, transmitter-gated channels are similar to the acetylcholine receptor. Mechanisms to generate an excitatory synaptic potential (with a transmitter-gated receptor/channel) are similar to that of generating an action potential (with voltage-gated channels) in that both involve movement of sodium and potassium ions across the membrane. They differ in several ways, however:
- In the generation of action potentials, sodium and potassium ions move through different voltage-gated channels - i.e. the channels are selective for the ion. Transmitter-gated channels usually are not selective for the ions since the pore is too large.
- ion flux through voltage-gated channels is "regenerative" in that increased depolarization caused by the channel opening leads to more influx of the ion. In transmitter-gated channel, the amount of ion passing through the membrane depends on the local concentration of neurotransmitter released at the synapse.
- When acetylcholine causes the opening of the acetylcholine receptor/pore, sodium can flow in and potasium can flow out (down their respective concentration gradients). However, more sodium flows in initially than potassium flows out since potassium efflux is resisted by the negative transmembrane potential. This leads to an inward flow of sodium enough to cause the activation of potential-gated sodium channels. Since these are voltage gated, much more sodium can enter the cell, leading to an action potential and the ultimate opening of vesicular calcium ion channels. This leads to a rise in intracellular calcium (through opening of a Ca channel in membranes of intracellular organelles with high concentrations of Ca ions), which activates muscle contraction.
![]() Electron
Micrograph of Junction-End Plate
Electron
Micrograph of Junction-End Plate
![]() Animation:
Acetylcholine receptor
Animation:
Acetylcholine receptor
![]() Updated
Acetylcholine Receptor Pore
Jmol14 (Java) |
JSMol (HTML5) (1OED)
Updated
Acetylcholine Receptor Pore
Jmol14 (Java) |
JSMol (HTML5) (1OED)
![]() How
does ligand binding lead to channel opening? This has been studied in
detail by Douherty and Lester for binding of acetylcholine, a positively
charged ligand to its neural receptor, the neuronal nicotinic nACh receptor,
a ligand-gated ion channel. It, along with the neural serotonin (HT3)
and GABA receptors belong to the
Cys-Loop superfamily of receptors which consists of 5 protein chains.
Douherty and Lester have reconstituted the AChR into Xenopus oocyte (egg)
membranes and have done a combination of electrophysiological and chemical
studies to probe the conformational changes in the receptor. In the
absence of ligand the channel pore is blocked by 5 leucine side chains,
which move on ligand binding. The neurotransmitter binds to a
tryptophan side chain in an "aromatic box" that contains five aromatic amino
acids), with the positive charge interacting with the aromatic cloud by
cation-π interactions. Serotonin and benzodiazepines (a ligand
that interacts with the GABA channel - see below) bind to different
aromatic side chains in the aromatic boxes in their receptors, indicating
much less specific interactions compared to enzyme-substrate interactions.
This explains how nicotine can bind to neural AChR and activate it even
though their structures differ significantly. They found that the
conformational changes allowing channel opening requires a trans-cis
isomerization of a Pro side chain. Site-specific mutants that favor
the trans form favor closing. However, not all members of the Cys-Loop
superfamily have a Pro at this position.
How
does ligand binding lead to channel opening? This has been studied in
detail by Douherty and Lester for binding of acetylcholine, a positively
charged ligand to its neural receptor, the neuronal nicotinic nACh receptor,
a ligand-gated ion channel. It, along with the neural serotonin (HT3)
and GABA receptors belong to the
Cys-Loop superfamily of receptors which consists of 5 protein chains.
Douherty and Lester have reconstituted the AChR into Xenopus oocyte (egg)
membranes and have done a combination of electrophysiological and chemical
studies to probe the conformational changes in the receptor. In the
absence of ligand the channel pore is blocked by 5 leucine side chains,
which move on ligand binding. The neurotransmitter binds to a
tryptophan side chain in an "aromatic box" that contains five aromatic amino
acids), with the positive charge interacting with the aromatic cloud by
cation-π interactions. Serotonin and benzodiazepines (a ligand
that interacts with the GABA channel - see below) bind to different
aromatic side chains in the aromatic boxes in their receptors, indicating
much less specific interactions compared to enzyme-substrate interactions.
This explains how nicotine can bind to neural AChR and activate it even
though their structures differ significantly. They found that the
conformational changes allowing channel opening requires a trans-cis
isomerization of a Pro side chain. Site-specific mutants that favor
the trans form favor closing. However, not all members of the Cys-Loop
superfamily have a Pro at this position.
In a very recent finding, Dougherthy's group discovered the mechanism underlying the fact that nicotine does not bind with high affinity to the acetylcholine receptor in muscles. Apparently it can not make the cation-π interactions necessary for interaction with the receptor. A H-bond between a H attached to the positive charged nicotine to the Trp carbonyl in the aromatic box allows the cation-π interaction in the neural form. The H bond is not apparently not present in the muscle form, which prevents strong cation-π interaction with nicotine. If it could bind to the muscle acetylcholine receptor, nicotine would be so poisonous that no one would smoke.
Navigation
Return to Chapter 9B: Neural Signaling Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9B: Neural Signaling

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.