Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 9 - SIGNAL TRANSDUCTION
B: NEURAL SIGNALING
BIOCHEMISTRY - DR. JAKUBOWSKI
06/10/14
|
Learning Goals/Objectives for Chapter 9B:
|
B8. Voltage-Gated Potassium Channel
How can the two essential features of the K+
channel pore - selectivity for the larger potassium ion over the sodium ion,
and the near diffusion-limited rate of potassium ion flux through the
channel be reconciled?
Figure: Electrostatics of the K+ channel .
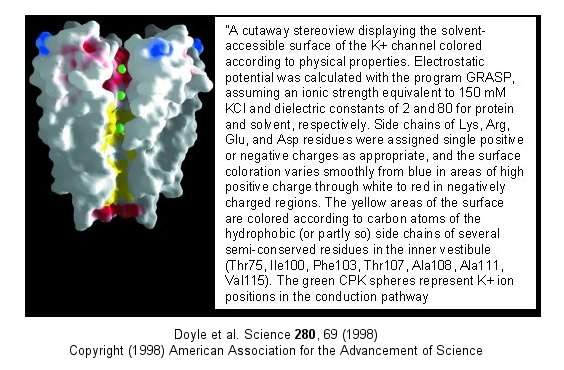
The selectivity filter is composed of many stacked rings of oxygens
that can interact with a dehydrated K ion but not with a dehydrated Na ion
which can not approach close enough to form significant interactions.
Surrounding the filter are twelve aromatic amino acids which constrain the
size of the pore opening. The interactions of the filter O's with the
K ion makes up for the energetically disfavored dehydration of the ion.
The filter contains two K ions which repel each other, assisting in
the vectorial discharge of the ions through the membrane. These ions
must form weak interactions with the selectivity filter. The actual
pore is mostly hydrophobic, which facilitates flow-through of the ions.
The central cavity of the pore can hold some waters molecules in addition to
the K ions which helps stabilize the ion in the middle of the pore.
In another recent paper (Nature, 404, April 20, 2000, 881-884), molecular dynamic calculation were performed to distinguish between various models for binding and selectivity of the channel. They found the favored conduction pathway arises from conformational changes between 2 discrete shapes that differ in energy by only about 5 kcal/mol (about 1-2 H bonds). The picture below shows the channel with one of the 4 subunits removed for clarity. Water is shown in red and the K ions in blue. Two ions are in the filter and one in the middle cavity.
Figure: K+ Channel from Steptomyces lividans
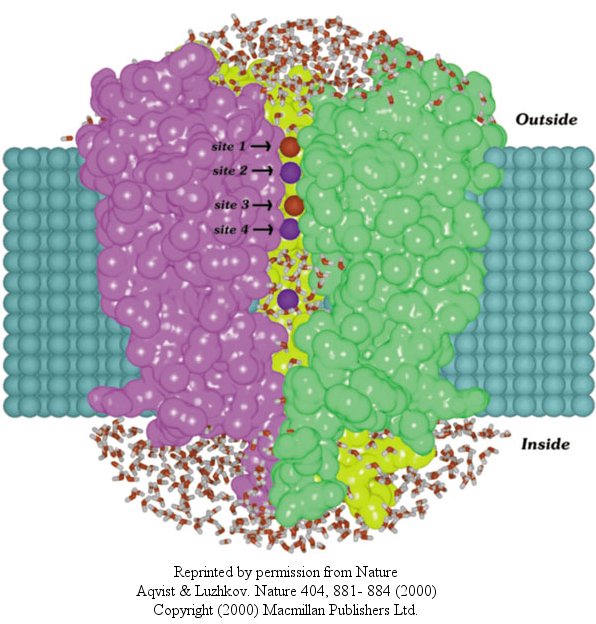
![]() Jmol:
Potassium Channel Not updated; Needs work
Jmol:
Potassium Channel Not updated; Needs work
MacKinnon's laboratory (2003) has recently determined the X-ray structure of a voltage-dependent K+ channel from Aeropyrum pernix, an archaebacteria. This protein is homologous to eukaryotic K+ channels. This membrane protein was solubilized with detergent and crystallized in the presence of an antibody which inhibited the flexibility of part of the protein, facilitating the formation of crystals. Like the sodium channel, this protein has 4 subunits each consisting of a 6 alpha-helices which cross the membrane. The S4 helix, which has multiple positively charged side chains, is present in each subunit and acts as a voltage sensor. It was presumed that the helices would would twist in a ratchet-like fashion, leading to the movement of positive charge in the S4 helix across the membrane (as described above for the Na channel). MacKinnon's group found evidence for another mechanism for voltage sensing. Crystal structures show that the S4 helices is imbedded in the membrane, and is not interacting directly with other parts of the protein. It forms an alpha-helical haripin (a paddle shape) which can move through the membrane on change of the transmembrane potential. The topological disposition of paddle (within the membrane) explains the effect of small, lipophilic molecules (like anesthetics nerve toxins, insecticides like DDT) on the channel. His group also labeled the S4 helix with a biotin-17 angstrom linker complex. On change of the transmembrane potential the biotin flips from across the membrane where it can bind to (and be detected by) its natural binding partner, avidin. The authors suggest that the "voltage sensitive paddles operate somewhat like hydrophobic cations attached to levers, enabling the membrane electric field to open and close the pore."
Figure; S4 paddle voltage sensor of the voltage-dependent K+ channel from Aeropyrum pernix
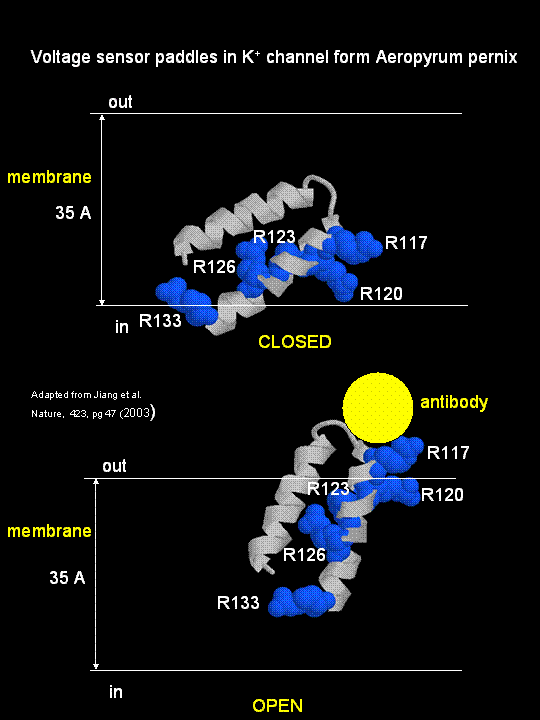
Channels, once open, must be inactivated. In the case of the voltage-gated potassium channel, inactivation occurs when the amino-terminal cytoplasmic domain binds to the potassium pore on the cytoplasmic side, in interaction likened to the binding of a "ball on a chain" (the cytoplasmic domain) to the pore opening. The chain acts to tether the ball domain so it may swing to bind to the pore opening. The ball domain contains both positively charged and hydrophobic regions. Where is the ball domain in the absence of inhibition? Recent studies (Oliver et al.) have shown that a positive domain can bind to proximal phosphatidyinositol 4,5-bisphosphate (PIP2) lipids on the inner leaflet of the membrane bilayer. When so bound, inactivation of the channel is prevented. As you will see in the next section, PIP2 can also be cleaved to form diacylglycerol and inositol 1,4,5-trisphosphate when cells are activated byexternal factors (hormones, growth factors, etc) in the process of signal transduction.
The latest (2005) new structure (Long et al) from MacKinnon's group of the another eukaroytic K+ channel (in its open state) is more consistent with previous expectations. Instead of using antibodies to stabilize the voltage-sensor domains, they crystallized the physiological channel, which in eukaryotes consists not only of 4 identical membrane spanning subunits, but also an addition domain T1 and another protein, beta. The T1 domain consists of the N terminal regions preceding the first membrane-spanning helix, S1, of each of the four monomers of the channel and is located intracellularly at the entrance point of the channel. Four conserved Arg resides on S4, part of the voltage-sensor domains, are hypothesized to move under the influences of forces arising from changes in the membrane's electric field initiated by ion movement through other ion channels in the membrane. Mechanical work is done by the electric field on the voltage sensor as the charged Arg residues are moved through the electric field and the protein conformation is altered. In turn, the S4 and coupled S5 helices of the voltage sensor do mechanical work on the pore by altering its conformation to open/close the pore, specifically at the activation gate of the pore. This seems quite similar to how iron movement into the heme plane in hemoglobin on oxygenation pulls the proximal His on the F8 helix which then transmits a conformation change to other helices in the subunit, leading to cooperative conformational changes in the tightly packed protein. Two of the Arg residue on the S4 sensor are facing the lipid interface while the other two are buried in the voltage-sensor domain. Their work shows that this domain is clearly separate from the pore domain, much as the extracellular ligand-binding domain of neurotransmitter-gated ion channels are separated from the pore domain. In this case, however, the voltage-sensor domain is buried in the membrane.
![]() Updated
Mammalian Voltage-Dependent Shaker Family Potassium Channel
Jmol14 (Java) |
JSMol (HTML5)
Updated
Mammalian Voltage-Dependent Shaker Family Potassium Channel
Jmol14 (Java) |
JSMol (HTML5)
Alterations of potasium channels has recently been implicated in the inhibitory effects of ethanol. Davies, McIntire, et al. studied effects of ethanol on the round worm C. elegans, believing that alcohol-mediated inhibition of neural activity would be conserved across species. Previously it has been shown that the dose-effect curves for behavioral changes is similar for both invertebrates and vertebrates. Ethanol seems to affect many different proteins that would lead to synaptic inhibition, including GABA and glutamate channels and potassium channels. Many different gene products (dopamine D4 receptors, protein kinase C) are associated with increased sensitivity while others (nitric oxide synthase, dopamine D2) are associated with ethanol resistance. These changes suggests the complex pathways are involved in ethanol effects but don't isolate a specific target for its effects.
When C. elegans were exposed to ethanol for brief time periods, ethanol levels rose to values similar to levels seen in intoxicated drivers (0.1%). They isolated mutants that were resistant to ethanol effects (changes in movement and egg-laying behavior). These mutants affected a single gene, slo-1, homologous to the the slowpoke gene in drosophila. The gene in both organisms encoded a BK potassium channel, whose normal function is to repolarize neural membranes. Mutants strains, when transformed with slo-1+ regained ethanol sensitivity. Ethanol appears to directly activate the channel. This would lead to efflux of potassium ions from the worm, hyperpolarizing the neural cells, leading to inhibition of neural activity. Effects in C. elegans were observed at physiologically relevant ethanol, ranging from those that produce euphoria to sedation in humans.
Navigation
Return to Chapter 9B: Neural Signaling Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 9B: Neural Signaling

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.