CO12b. Enolate Addition: Aldol reactions
Aldol Reactions: Adding Enolates to Carbonyl Electrophiles
In the last section, we looked at enolate ions. We saw that enolate ions form when a proton is lost from the carbon next to a carbonyl. These ions are pretty stable because they are resonance stabilized. They are also pretty good nucleophiles.
-
Simple carbonyls are electrophiles.
-
The enolate ions that form from simple carbonyls are nucleophiles.
-
Carbonyl electrophiles react with enolate ion nucleophiles.
The reaction of an enolate nucleophile with another carbonyl compound is called an aldol reaction. Very often, the reaction occurs when a sample of an aldehyde or ketone is treated with a strong base such as sodium hydroxide. Most of the time, the molecules and the sodium hydroxide base are dissolved in an alcohol to get things mixed together. In a flask or vial containing a solution of this compound, there will be millions of aldehyde or ketone molecules. Some of those molecules become deprotonated and turn into enolate nucelophiles. Some of them remain as carbonyls and act as electrophiles. The molecules pair off together and react, bonding to each other to make molecules that are twice as big as the ones we started with. A simple example of this reaction is shown here. This example involves the reaction of 2-propanone with its enolate.
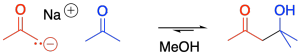
Figure CO12b.1. An aldol reaction.
Note the pattern in the product. The carbonyl of the enolate is connected to the enolate carbon which is connected to the alcohol carbon. The alpha carbon of the nucelophile connects one carbonyl to another carbonyl, but the second carbonyl has become an alcohol.
- In an aldol reaction, an enolate nucleophile undergoes addition to a second aldehyde or ketone.
- The product contains the strutural fragment O=C-C-C-OH: three carbons in a row, with a double bond to oxygen on the forst and an OH group on the third.
Problem CO12b.1.
Provide a mechanism, with curved arrows, for the aldol reaction of 2-propanone, above.
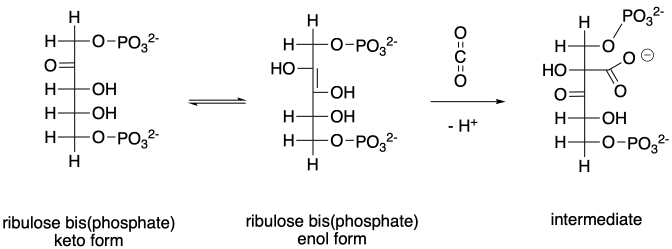
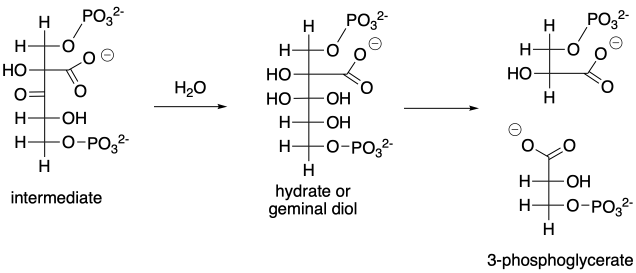
The biosynthesis of sugars, such as fructose, involves coupling smaller sugars together. If one sugar is converted into a nucleophile, it can donate electrons to the carbonyl on the other sugar, forming a new C-C bond. The carbonyl on the second sugar becomes a hydroxyl group in the new, larger sugar.
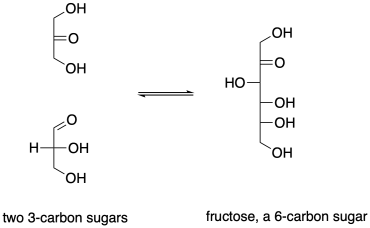
Figure CO12b.2. An aldol reaction in the formation of fructose.
In the cell, sugars are typically in a phosphorylated form when they react in this way. Phosphorylation is often an important step in activating molecules for biochemical reactions.
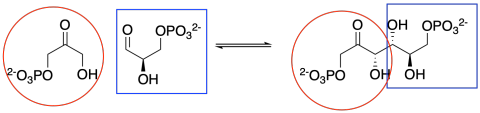
Figure CO12b.3. Keeping track of the precursors in fructose formation.
Problem CO12b.2.
Show the mechanism for the formation of the phosphorylated fructose shown above.
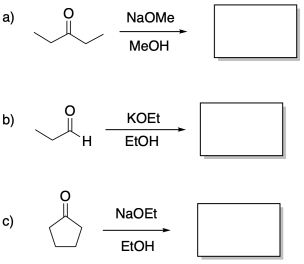
Problem CO12b.3.
Predict the products of the following aldol reactions.
Sometimes, two different compounds may react together in an aldol reaction. One compound acts as the nucleophile, and the other one acts as the electrophile. However, the reaction is really not much different that the aldol reactions we have already seen.
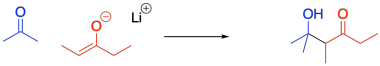
Figure CO12b.4. A crossed aldol reaction.
The only complication is that now there are two different compounds that could potentially be nucleophiles and two different compounds that could potentially be electrophiles. That makes predicting the outcome of the reaction a little more difficult.
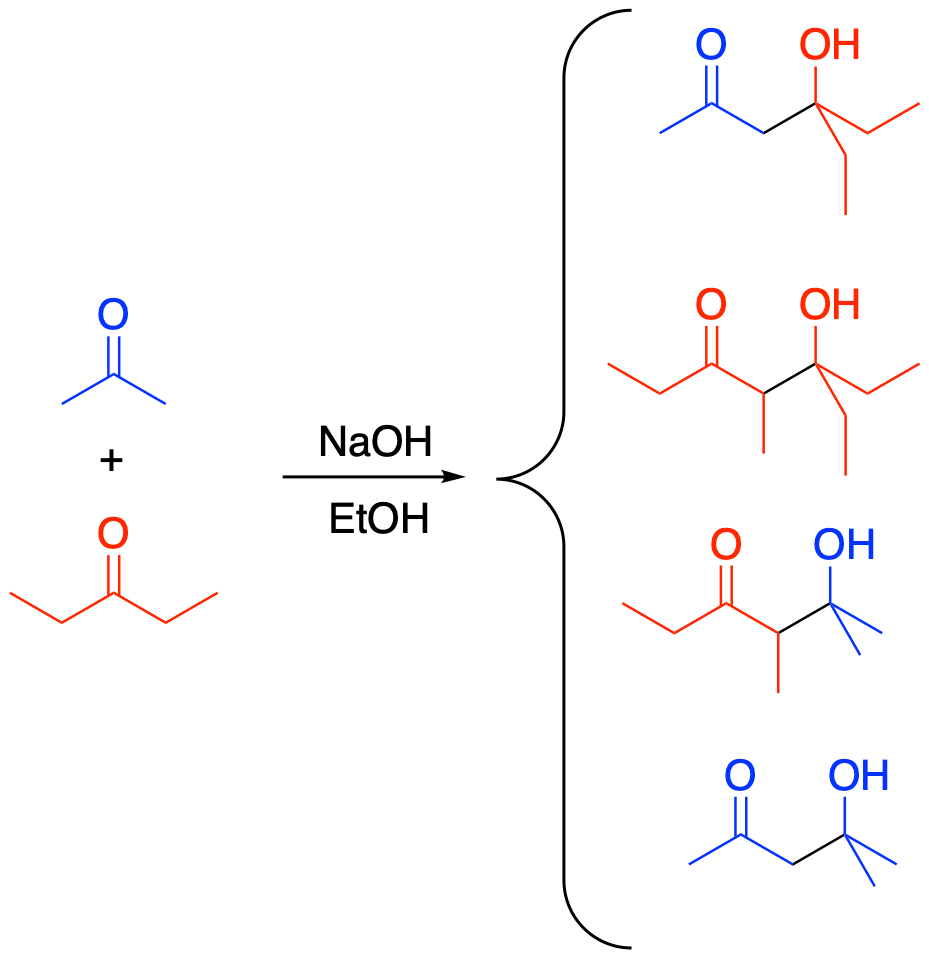
Figure CO12b.5. A crossed aldol reaction leading to a mixture of four different products.
- Aldol reactions can happen between two different carbonyl compounds.
- Multiple products are possible, depending which compound forms the enolate nucleophile and which acts as the electrophile.
Problem CO12b.4.
The following compounds would give multiple products through different aldol addition reactions. Show the products.
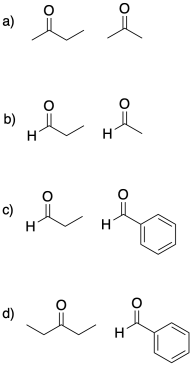
There are cases where it becomes a lot more obvious which compound would be the electrophile and which one would be the nucleophile. Maybe one of the compounds has a carbonyl that is much less crowded than the other. For example, maybe one compound is an aldehyde and the other one is a ketone. The less crowded carbonyl, the aldehyde, is much more likely to act as a good electrophile.
Maybe one of the compounds doesn't even have any alpha-protons. In that case, it can't be deprotonated and it can't form an enolate anion. It won't be able to act as the nucleophile.
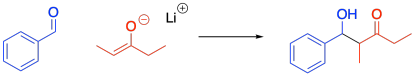
Figure CO12b.6. A more reliable crossed aldol reaction.
- Crossed aldol reactions can give a single major product if one of the partners has no alpha protons.
- That compound can't become an enolate nucleophile, so it acts only as an electrophile.
- If that compound is an aldehyde, it will be a much better electrophile than a ketone partner.
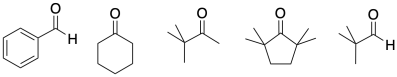
Problem CO12b.5.
Only some of the following compounds may undergo aldol reactions. Select which ones may not undergo the reaction, and explain what factor prevents them from reacting.
There is another, more general method of running an aldol reaction using two different ketones for the nucleophile and the electrophile. This method is called a directed enolate reaction. We are trying to direct one molecule to become the enolate nucleophile and the other molecule to be the carbonyl electrophile. We do that by being careful about the order in which we add things to the reaction. If we start with the compound that we want to turn into the enolate nucleophile, and we add to that a very strong base, such as lithium diisopropylamide (LDA), then we should get 100% formation of the enolate ion (or close enough). Second, we add the other ketone to act as the electrophile. We run this reaction in an aprotic (think non-hydrogen bonding) solvent so that there are no protons that might reprotonate the enolate before we are ready. Third, we add an acid to quench the reaction and neutralize the product.

Figure CO12b.7. A directed aldol reaction.
- Crossed aldol reactions can also work if one carbonyl compound is completely converted into enolate ion before the other partner is added.
- The first compound acts as the nucleophile.
- The carbonyl compound added second acts as the electrophile.
Aldol reactions do not just occur with enolate anions, however. Enols are the neutral form of enolates, protonated on the oxygen instead of the alpha carbon. Enols are also good nucleophiles. In an enol nucleophile, the pi bond acts as the electron source, rather than the lone pair. However, the pi bond gets a boost from the lone pair on the oxygen.
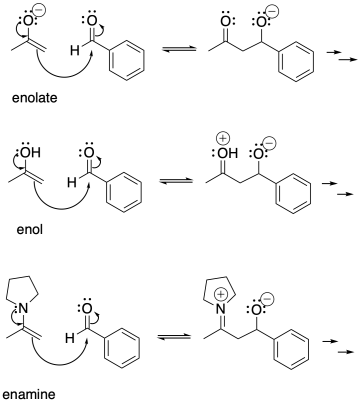
Figure CO12b.8. Enolates, enols, and enamines all add to carbonyls.
Enols are always present in equilibrium with aldehydes and ketones. An enol is a simple tautomer of a carbonyl compound. To get from one to the other, a proton is simply transferred from one position to the other.
-
Enamines and enols are also good nucleophiles for aldol reactions.
-
Because either acid or base can catalyse keto-enol tautomerism, aldol reactions can be catalysed by either acid or base.
There is another important variation of aldol reactions in addition to these ones. Aldol condensations can also result in loss of a water molecules to produce and "enone". That's an addition product in which the OH group produced in the aldol addition is replaced by a carbon-carbon double bond. We'll look at this variation in the next section.
Problem CO12b.6.
Show the subsequent protonation step in the reactions involving the enol and the enamine above.
Problem CO12b.7.
An enamine reaction is usually followed by hydrolysis of the C=N bond in the iminium ion. Show the mechanism for conversion of the iminium ion to the carbonyl via addition of aqueous acid.
Problem CO12b.8.
Which conditions would be most likely to form this aldol product (select a, b, c or d below)?
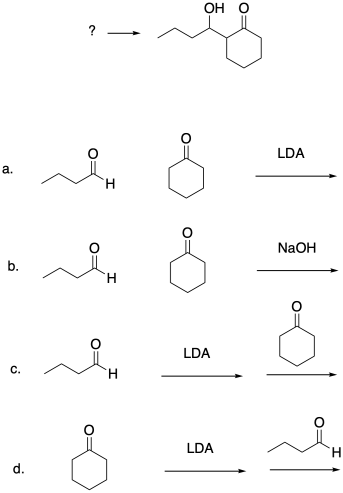
Problem CO12b.9.
Provide products of the following acid-catalysed aldol reactions.
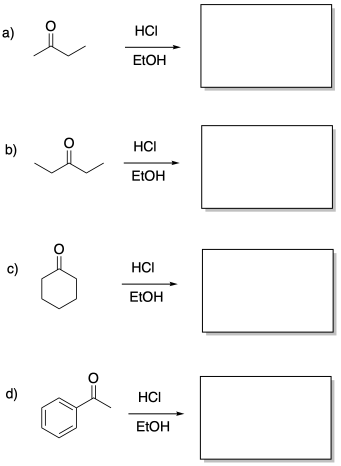
Problem CO12b.10.
Draw the enol nucleophiles for the above question.
Problem CO12b.11.
Provide the products of the following enamine additions, after hydrolysis.
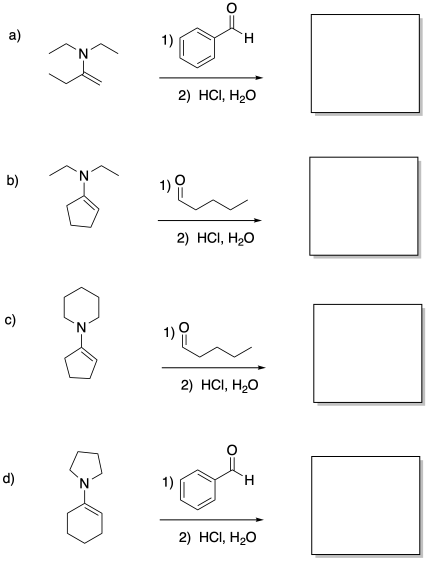
Problem 12b.12.
Identify the reactants that would make these products via aldol addition.
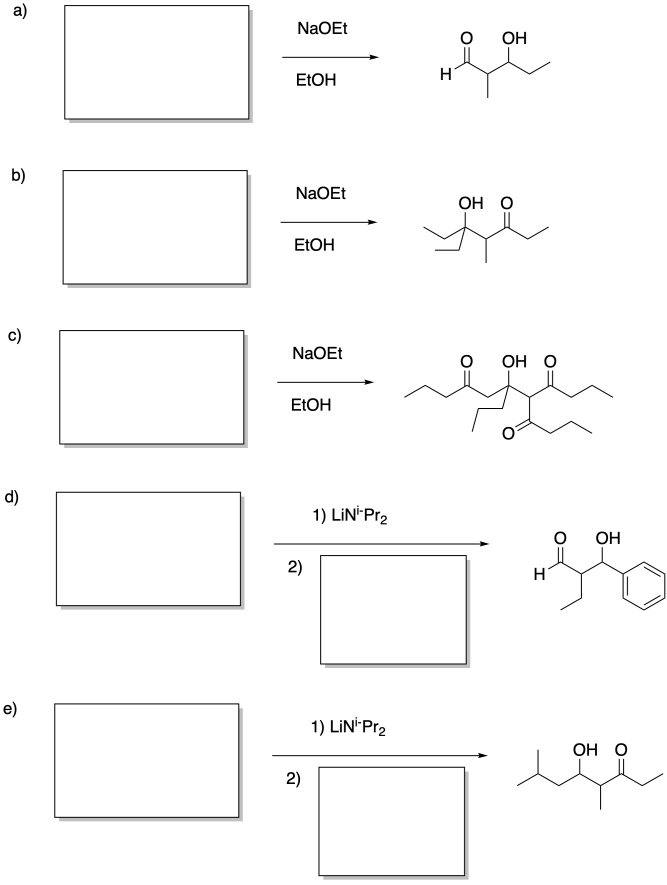
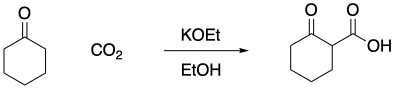
Problem CO12b.13.
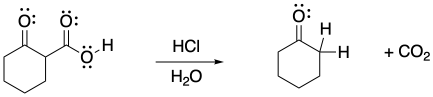
Carbon dioxide can act as the electrophile in an aldol reaction. Provide a mechanism for the reaction below.
Problem CO12b.14.
Aldol reactions can occur in equilibrium; they can be reversible. Aldol reactions in reverse are generally called "retro-aldol" reactions. The aldol reaction with carbon dioxide is usually reversed under acidic conditions, via enol instead of enolate ion. Because the reaction results in loss of carbon dioxide, it is called a "decarboxylation" reaction.
Provide a mechanism for the decarboxylation reaction below.