CO12c. Enolate Addition: Aldol Condensations
We have already looked at how aldol addition reactions result from the combination of an enolate anion nucleophile with an aldehyde or ketone electrophile. Sometimes, aldol reactions are followed by a subsequent reaction, called an elimination reaction. That reaction formally produces a molecule of water. Early studies of this reaction would result in droplets of condensation on the glassware in which the reaction occured; hence, it is sometimes called a condensation reaction.
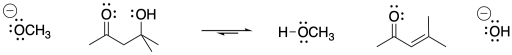
Figure CO12c.1. An aldol condensation: elimination of water from an aldol product.
Notice the structural pattern in the product. An aldol condensation leads to the formation of a C=C-C=O group. This group is often called an enone.
The term "condensation" comes from the fact that the reaction formally results in loss of a water molecule from the alpha and beta positions (H from alpha and OH from beta). Early reactions that were observed to result in loss of water were frequently described as condensations because of the water droplets that would appear on the glassware (literally, condensation) as the reaction proceeded. The term "dehydration" is also used to describe this loss of water.
However, don't get too tied to the descriptions of the reactions (condensation vs. addition). The terms are used loosely and sometimes interchanged. On this page, we'll try to use addition to describe the initial product and condensation to describe the product after loss of water, but sources elsewhere might not describe it that way.
Problem CO12c.1.
Provide a mechanism for the dehydration step in the aldol condensation shown above.
It can be hard to predict the outcome of an aldol reaction because of the fact that there are two possible products from an aldol reaction (one with a new hydroxyl and one with a new double bond). A chemist might try to make one product in the laboratory, and end up with the other. This process can be difficult to control. However, in general, the elimination reaction is encouraged by heating the reaction. The reaction sometimes occurs without elimination if the reaction is kept cool.
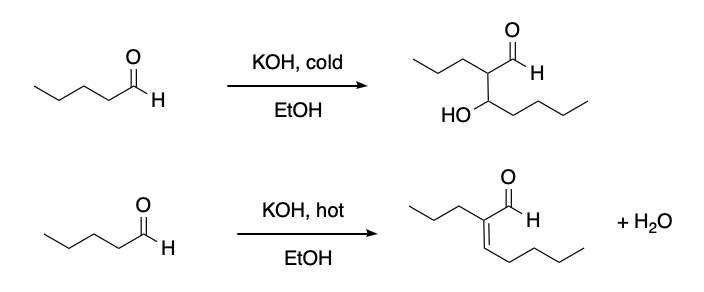
Figure CO12c.2. Aldol addition vs. condensation depends on heat.
Why does heating the reaction make the condensation part of the reaction more likely to happen? Mostly it comes down to entropy. The simplest way to evaluate entropy change in a reaction is to determine whether the number of particles or molecules is increasing during the reaction, decreasing, or staying the same. If the number of molecules is increasing, then entropy also increases. That's favourable and it helps drive the reaction forward. If the number of molecules is decreasing, then entropy is also decreasing. That's unfavourable and it helps drive the reaction backward. If the number of molecules stays the same before and after the reaction, then the entropy change is usually pretty small.
Heat plays a role that is illustrated by Gibbs Free Energy:
ΔG = ΔH - TΔS
The Gibbs free energy relationship says that the effect of entropy is multiplied by the temperature; in other words, entropy becomes the dominant thermodynamic factor at higher temperatures.
- Aldol condensations, with removal of water, are promoted by heat.
- Colder temperatures can result in aldol addition reactions.
Just as in regular aldol additions, aldol condensations become more complicated if two different carbonyl compounds are used. Remember, that's because it may be hard to predict which one acts as the nucleophile and which acts as the electrophile. In the case of asymmetric ketones, just one compound can lead to multiple products, because the enolate formed when a proton is removed from one side of the carbonyl is different from the enolate formed when a proton is removed from the other side. That means there are two different possible nucleophiles.
Furthermore, because a double bond is formed in the condensation reaction, there is the possibility of forming either the (E) product or the (Z) product. However, we would expect to get the sterically less crowded of those possibilities as a major product. That's because the sigma bond rotation would allow the larger groups to be further apart from each other already by the time the loss of water occurs.
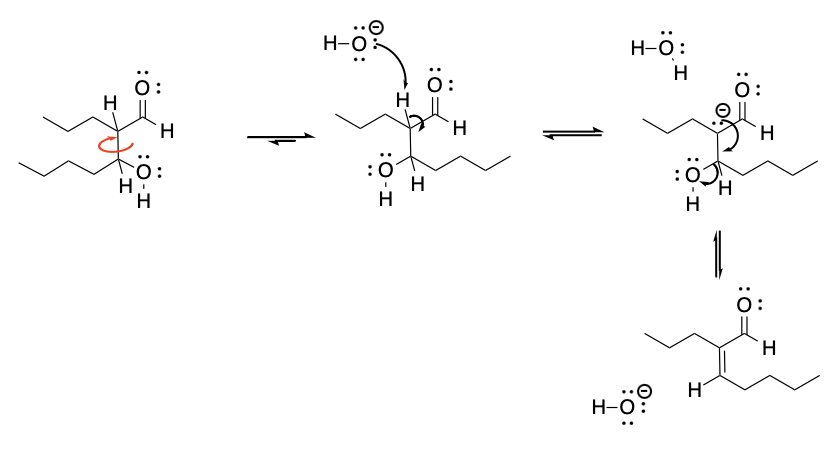
Figure CO12c.3. Conformational adjustments before elimination allow the formation of the more stable trans double bond.
Problem CO12c.2.
Treating 2-butanone with strong base and heat can potentially give a mixture of four aldol condensation products, taking into consideration different α-protons and cis/trans isomers. Draw the four possible products.
Once again, things are much simpler if one compound is an aldehyde without any alpha protons and the other compound is a ketone. That way, one compound is reliably the electrophile and the other compound is turned into the nucleophile.
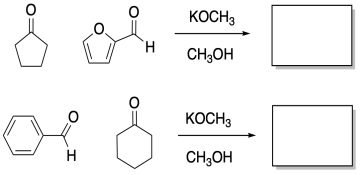
Problem CO12c.3.
Fill in the products of the following aldol condensations.
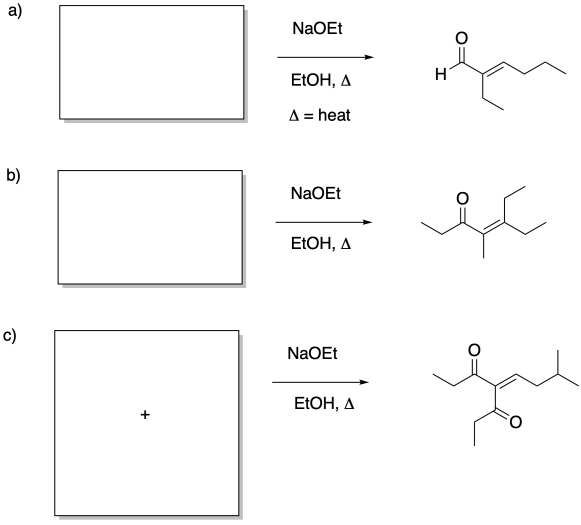
Problem CO12c.4.
Identify the reactants that would make these products via aldol addition.
Solutions to selected problems
This site was written by Chris P. Schaller, Ph.D., College of Saint Benedict / Saint John's University (retired) with other authors as noted on individual pages. It is freely available for educational use.

Structure & Reactivity in Organic, Biological and
Inorganic Chemistry by
Chris Schaller is licensed under a
Creative
Commons Attribution-NonCommercial 3.0 Unported License.
Send corrections to cschaller@csbsju.edu
This material is based upon work supported by the National Science Foundation under Grant No. 1043566.
Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Navigation:
Back to Carbonyl Addition Index
Back to Web Materials on Structure & Reactivity in Chemistry