Biochemistry Online: An Approach Based on Chemical Logic

CHAPTER 8 - OXIDATION/PHOSPHORYLATION
B: OXIDATIVE ENZYMES
BIOCHEMISTRY - DR. JAKUBOWSKI
04/15/16
|
Learning Goals/Objectives for Chapter 8B: After class and this reading, students will be able to
|
B8. OXIDASES
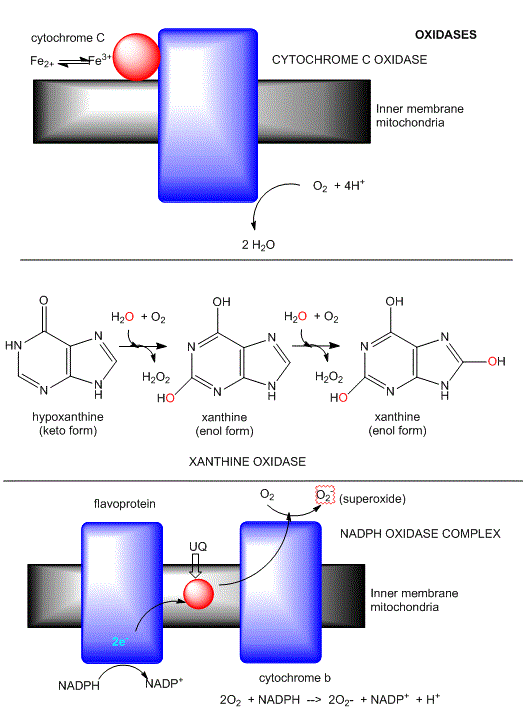
This class of enzymes does not incorporate dioxygen into an organic substrate. Rather it accepts electrons released from an organic substrate, through intemediate electron carriers (such as ubiquinone and cytochrome C) to form superoxide (as in NADPH-oxidase), hydrogen peroxide (as in xanthine oxidase) or water (as in cytochrome C oxidase). The mechanism of cytochrome C oxidase again supports our expectations about enzymes that use dioxygen. Dioxygen binds metals in the enzyme. One oxygen atom binds a heme Fe2+ of cytochrome a3 which is bound to the enzyme, while the other binds a Cu1+ of Cu B. All oxygyen reduction intermediates remain bound to the enzyme. Four electrons are added from four different cytochrome C molecules, which serve as mobile carriers of electrons.
![]() Jmol: Updated
Cytochrome C Oxidase
Jmol14 (Java) |
JSMol (HTML5)
Jmol: Updated
Cytochrome C Oxidase
Jmol14 (Java) |
JSMol (HTML5)
Figure: Oxidases: Examples
Another example of an oxidase is monoamine oxidase. Mitochondrial monoamine oxidase catalyzes the oxidative deamination of certain neurotransmitters after they have been taken up by post-synaptic neurons, in a process of inactivation. A reaction is shown below.
Figure:
Monoamine oxidases
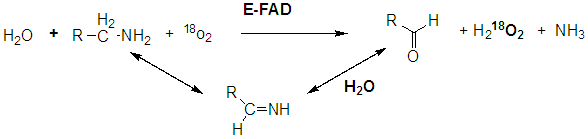
A Schiff base is formed which is then hydrolyzed, incorporating unlabeled oxygen into the oxidized molecule.
BIOLOGICAL OXIDATIONS: METHANE TO CO2
In the previous chapter section, we discussed the progressive oxidation of methane by 2 electron loses to form methanol, formaldehyde, formic acid and CO2, with a progressive increase in oxidation number for the C by +2 (from -4 in methane to +4 in CO2).
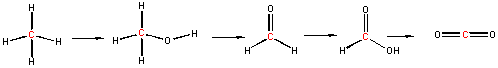
Methanotrophs are aerobic bacteria that use methane as a source of energy, converting it in a series of two electron oxidations as shown above, to carbon dioxide. The enzymes involved in this sequential process are methane monooxygenase, methanol dehydrogenase, formaldehyde dehydrogenase, and formate dehydrogenase. Methane monoxygenase exists in a soluble and membrane form, both of which are part of a larger complex. Both have a hydoxylase (which uses dioxygen to add O to methane) and the membrane form has recently been shown to be associated with have methanol dehydrogenase in a larger complex consisting of trimers of each enzyme (the hydroxylase and the dehydrogenase).
Navigation
Return to Chapter 8B: Oxidative Enzymes Sections
Return to Biochemistry Online Table of Contents
Archived version of full Chapter 8B: Oxidative Enzymes

Biochemistry Online by Henry Jakubowski is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.